Research Articles
Advanced Strategies for Enhancing Protein Solubility and Stability: From Molecular Design to Clinical Application
This article provides a comprehensive analysis of contemporary strategies for enhancing protein solubility and stability, critical factors in biotherapeutic development and research applications.
Strategies for Improving Thermal Stability of Vaccine Immunogens: From Molecular Design to Thermostable Formulations
This article provides a comprehensive review of the latest scientific and technological advancements aimed at enhancing the thermal stability of vaccine immunogens.
Strategies for Troubleshooting and Optimizing Low Protein Expression in Heterologous Hosts
This article provides a comprehensive guide for researchers and scientists facing challenges with low or no protein expression in heterologous systems like E.
Optimizing Protein Concentration Measurement: A Guide to Enhanced Accuracy for Reliable Research and Development
Accurate protein quantification is a foundational pillar of research and drug development, yet it is challenged by the lack of a universal gold standard and numerous potential interferences.
Addressing Proteolysis in Protein Purification: From Workflow Challenges to Targeted Degradation Technologies
This article provides a comprehensive analysis of proteolysis in protein workflows, addressing both the challenge of unwanted protein degradation during purification and the opportunity of intentional proteolysis for therapeutic purposes.
Strategic Approaches to Reduce Endotoxin Contamination in Recombinant Proteins: From Prevention to Removal
This comprehensive article provides researchers, scientists, and drug development professionals with advanced strategies for controlling endotoxin contamination throughout recombinant protein production.
Optimizing Protein Homogeneity and Dispersity: A Comprehensive Guide for Reproducible Research and Drug Development
Achieving optimal protein homogeneity and dispersity is a critical, yet often challenging, prerequisite for reproducible research in biochemistry, structural biology, and drug development.
Strategies for Enhancing Marginal Protein Stability in Heterologous Expression Systems
This article provides a comprehensive overview of advanced strategies for improving the marginal stability of recombinant proteins, a critical bottleneck in heterologous expression for biomedical research and drug development.
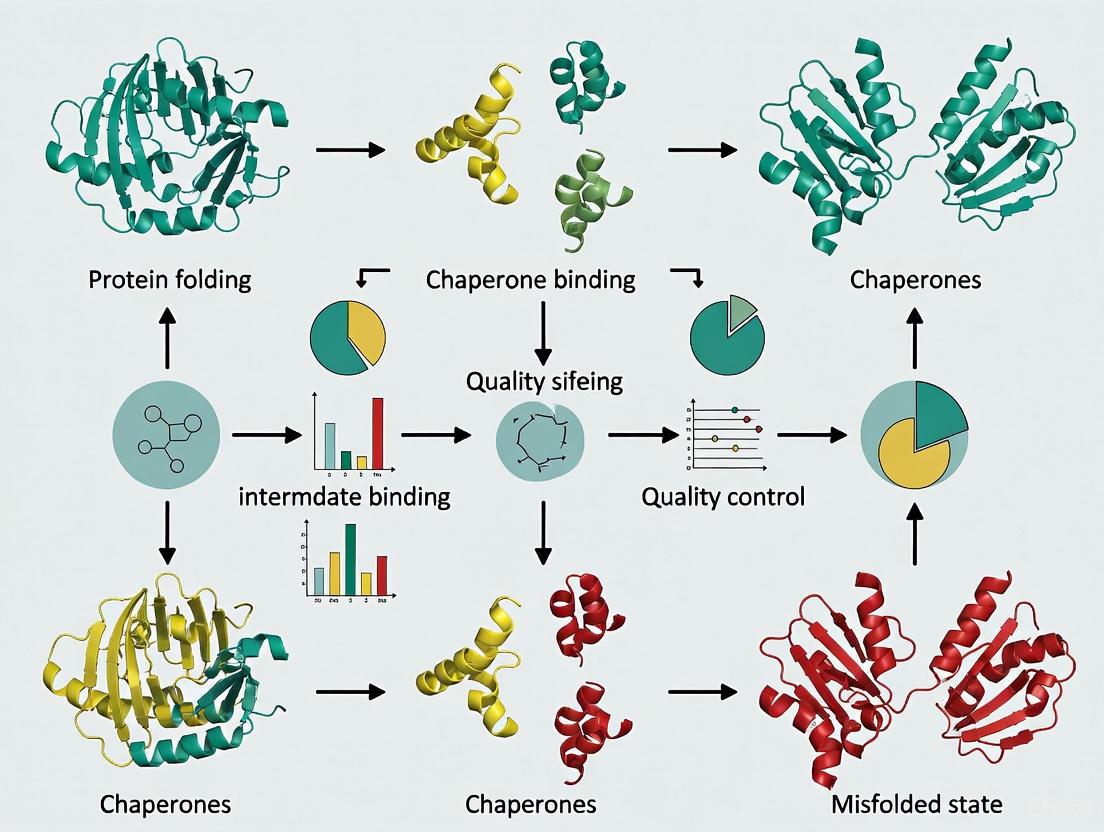
Chaperone-Assisted Protein Refolding: Protocols, Mechanisms, and Clinical Applications
This article provides a comprehensive guide to chaperone-assisted protein refolding, bridging foundational principles with practical laboratory applications.
Essential Quality Control for Recombinant Proteins: A Practical Guide to Improve Research Reproducibility
This article provides a comprehensive framework for implementing minimal quality control (QC) tests for recombinant protein samples, targeting researchers, scientists, and drug development professionals.