A Comprehensive Guide to Quality Assessment for Membrane Protein Preparations: From Bench to Validation
High-quality membrane protein preparations are fundamental to structural biology, functional studies, and drug discovery, with membrane proteins constituting over 60% of pharmaceutical targets.
A Comprehensive Guide to Quality Assessment for Membrane Protein Preparations: From Bench to Validation
Abstract
High-quality membrane protein preparations are fundamental to structural biology, functional studies, and drug discovery, with membrane proteins constituting over 60% of pharmaceutical targets. This article provides a systematic framework for the quality assessment of membrane protein preparations, addressing critical needs from foundational principles to advanced validation. It explores the biochemical and biophysical rationales behind quality control, details established and emerging methodological workflows for stability and function analysis, offers troubleshooting strategies for common pitfalls like aggregation and instability, and outlines rigorous validation techniques to ensure data reliability. Designed for researchers, scientists, and drug development professionals, this guide synthesizes current best practices to empower robust and reproducible membrane protein research.
Why Quality Matters: The Critical Role of Membrane Proteins in Health, Disease, and Drug Discovery
Membrane proteins are fundamental biological molecules embedded within cellular lipid bilayers. They are responsible for critical functions including signal transduction, molecular transport, and cell-to-cell communication. Their strategic location and diverse roles make them prime targets for therapeutic intervention; approximately 60% of current drugs target membrane proteins, with G-protein coupled receptors (GPCRs) alone accounting for about 27% of drugs on the market [1] [2]. Furthermore, their involvement in disease pathways positions them as valuable biomarkers for diagnosis and monitoring. This technical support center provides troubleshooting guides and FAQs to assist researchers in overcoming common challenges in membrane protein research, framed within the context of quality assessment for membrane protein preparations.
Troubleshooting FAQs
My membrane protein expresses poorly or not at all in a heterologous system. What should I check?
Poor expression is a common hurdle, often related to host cell toxicity, protein instability, or incorrect folding.
- Solution: Optimize Your Expression System
- Verify Vector and Sequence: Confirm your cloned sequence is correct and in-frame. Check for long stretches of rare codons that can cause truncation; use online tools for analysis and consider using host strains engineered to express rare tRNAs [3] [4].
- Change Your Competent Cells: Avoid standard BL21(DE3) cells for toxic proteins. Use specialized strains like C41(DE3), C43(DE3), or Lemo21(DE3), which have reduced background expression and gentler induction [5] [4]. For T7 systems, use strains expressing T7 lysozyme (e.g., pLysS or lysY) to suppress basal T7 polymerase activity [3] [4].
- Adjust Growth Conditions: Run an expression time course. Test different induction temperatures (e.g., 15-30°C) and inducer concentrations. Using a minimal growth medium (e.g., M9) can reduce the cell growth rate and improve the folding of some membrane proteins [5] [3].
- Consider a Solubility Tag: Fuse your target protein to a solubility tag like superfolder GFP or maltose-binding protein (MBP) to improve stability and expression yields [5] [4].
How can I extract my membrane protein while maintaining its stability and function?
The extraction process, which removes the protein from its native lipid environment, is a critical point where instability occurs.
- Solution: Choose and Optimize Your Solubilizing Agent
- Screen Detergents Systematically: Detergents are the most common solubilizing agents, but they can disrupt protein structure. Screen a variety of detergents (e.g., DDM, OG, Triton X-100) to find the optimal one. Use a concentration approximately 100x the detergent's Critical Micelle Concentration (CMC) [5]. Modern techniques like Flow-Induced Dispersion Analysis (FIDA) can screen multiple detergents rapidly with minimal sample volume [6].
- Use Membrane-Mimetic Systems: For functional studies, consider extracting with nanodiscs or lipid polymers. These systems engulf entire sections of the cell membrane, preserving the native lipid environment and oligomeric state of the protein, which is crucial for accurate functional assays [5] [7].
- Optimize Extraction Parameters: Allow sufficient time for extraction (3 hours to overnight) and perform it at a slightly elevated temperature (20-30°C) to increase efficiency, provided it does not harm your sample [5].
My membrane protein is insoluble or aggregates after purification. What can I do?
Aggregation often results from protein misfolding or the loss of structure-stabilizing lipids during extraction and purification.
- Solution: Stabilize the Protein Structure
- Re-evaluate Detergents: The detergent may be too harsh. Re-screen detergents or try mixtures with cholesterol homologs (CHS) to mimic the fluidity of native membranes [7].
- Use Chaperones and Lipids: Add lipids or chaperones to the purification buffer to assist with folding and stability [7]. For proteins requiring disulfide bonds, consider using engineered strains like SHuffle that facilitate correct bond formation in the cytoplasm [4].
- Lower Purification Temperature: Perform all purification steps at 4°C to slow down denaturation and aggregation [5].
- Reintegrate into a Lipid Bilayer: If using detergents for extraction, reintegrating the purified protein into artificial bilayer systems like nanodiscs can restore a more native environment and functionality [7] [6].
My protein binds poorly to the affinity chromatography column. How can I improve binding?
Low binding efficiency can be caused by the solubilizing agent masking the affinity tag or the tag being inaccessible.
- Solution: Enhance Tag Accessibility
- Use a Loose Resin: Instead of a static column, use loose affinity resin (e.g., loose Ni-NTA for His-tagged proteins) and mix it physically with your sample for several hours to encourage binding [5].
- Dilute Your Sample: Dilute the protein sample at least 2-fold to reduce the concentration of the solubilizing agent (detergent), which can act as a crowding agent and hide the affinity tag [5].
- Adjust the Affinity Tag: If the tag is buried, consider moving it to the opposite terminus of the protein or lengthening it (e.g., from 6xHis to 12xHis) [5].
- Change the Resin Metal: For His-tagged proteins, charging the resin with cobalt instead of nickel can increase purity, though it may reduce recovery [5].
Experimental Protocols for Quality Assessment
Protocol 1: Detergent Screening for Membrane Protein Solubilization
Objective: To rapidly identify the optimal detergent for solubilizing a membrane protein while maintaining its stability and monodispersity.
Materials:
- Purified membrane fraction containing your target protein.
- Detergent stock solutions (e.g., n-Dodecyl-β-D-maltoside/DDM, Lauryl maltose neopentyl glycol/LMNG, Octyl glucoside/OG, Triton X-100).
- Solubilization buffer (e.g., 50 mM Tris-HCl, 150 mM NaCl, pH 8.0).
- Thermonixer or water bath.
- Ultracentrifuge.
- SDS-PAGE equipment or FIDA instrument [6].
Method:
- Prepare Membrane Fraction: Isolate the membrane fraction from your expression host via cell lysis and differential centrifugation.
- Set Up Reactions: Aliquot the membrane fraction into multiple tubes. Add a different detergent to each tube at a concentration of 1-2% (w/v). Include a no-detergent control.
- Solubilize: Incubate the mixtures with gentle agitation for 3 hours at 4°C or 20°C.
- Separate: Ultracentrifuge the samples at high speed (e.g., 100,000 x g) for 30 minutes to pellet insoluble material.
- Analyze:
- Collect the supernatant (solubilized fraction) and analyze by SDS-PAGE to determine solubilization efficiency.
- For advanced analysis, use FIDA or dynamic light scattering (DLS) to measure the hydrodynamic radius and polydispersity of the solubilized protein, identifying conditions that yield a homogeneous, monodisperse population [6].
Protocol 2: Functional Characterization of a GPCR in Nanodiscs via Ligand Binding
Objective: To measure the binding affinity of a small molecule ligand to a GPCR reconstituted in nanodiscs under native-like conditions.
Materials:
- Purified GPCR in nanodiscs.
- Fluorescently labelled or unlabeled ligand.
- Binding assay buffer.
- FIDA instrument or Surface Plasmon Resonance (SPR) system [6].
Method:
- Reconstitute GPCR: Incorporate the purified GPCR into nanodiscs following established protocols to create a disc-like phospholipid bilayer encircled by a membrane scaffold protein.
- Prepare Ligand Dilutions: Create a dilution series of the ligand in the assay buffer.
- Measure Binding:
- Using FIDA: Mix the GPCR-nanodisc sample with each ligand concentration. FIDA measures the change in the hydrodynamic radius of the protein-ligand complex directly in solution, without the need for purification or immobilization. The shift in size is used to calculate binding affinity [6].
- Using SPR: Immobilize the GPCR-nanodisc on a sensor chip. Inject the ligand over the surface and monitor the binding response in real-time to determine kinetics and affinity.
- Data Analysis: Fit the binding data to an appropriate model (e.g., Langmuir isotherm) to determine the dissociation constant (Kd).
Research Reagent Solutions
Table 1: Essential Reagents for Membrane Protein Research
| Reagent Type | Example Products | Function & Application |
|---|---|---|
| Specialized Cell Lines | C41(DE3), C43(DE3), Lemo21(DE3), SHuffle T7 | Reduces basal expression for toxic proteins; promotes disulfide bond formation. [5] [4] |
| Detergents | n-Dodecyl-β-D-maltoside (DDM), Lauryl Maltose Neopentyl Glycol (LMNG) | Extracts proteins from the lipid bilayer; forms micelles around the protein. [5] |
| Membrane Mimetics | Nanodiscs, Liposomes | Provides a native-like lipid environment for structural and functional studies. [5] [6] |
| Affinity Chromatography Resins | Ni-NTA (Nickel/Nickel-Cobalt), Cobalt-Chelating Resins | Purifies recombinant proteins via affinity tags (e.g., His-tag). Cobalt can offer higher purity. [5] |
| Solubility Tags | MBP (Maltose-Binding Protein), superfolder GFP, Lysozyme tags | Enhances protein solubility and expression yields; allows tracking via fluorescence. [5] [4] |
| Protease Inhibitors | PMSF, EDTA-free cocktails | Prevents proteolytic degradation of target proteins during extraction and purification. [3] |
Table 2: Key Quantitative Data on Membrane Proteins and Assessment Tools
| Metric | Value or Statistic | Context & Significance |
|---|---|---|
| Market Share of Drugs | ~60% of drugs target membrane proteins [2]. | Highlights their immense pharmaceutical importance. |
| GPCR Drug Target Share | ~27% of all drugs target GPCRs [1]. | Underscores the dominance of one membrane protein family in pharmacology. |
| PDB Representation | <1% of PDB structures are membrane proteins [1]. | Explains the difficulty in computational modeling due to lack of templates. |
| IQ Scoring Function Success Rate | 93-100% in selecting native-like models [1]. | Demonstrates the high accuracy of dedicated model quality assessment programs. |
| HPMScore Performance | 46.9% success rate for Top 1 model selection [2]. | Outperformed DOPE (40.1%) in recognizing high-quality structural models. |
Signaling Pathways and Workflows
Diagram: KRAS Inhibitor Mechanism
Diagram: Membrane Protein Research Workflow
Technical FAQs: Core Stability Challenges and Mechanisms
FAQ 1: Why are membrane proteins inherently unstable outside of their native lipid bilayer?
Membrane proteins are unstable in aqueous solutions because their large hydrophobic transmembrane domains, which are normally stabilized by the lipid bilayer's hydrophobic core, become exposed to water. This exposure is energetically unfavorable and drives protein aggregation or denaturation [8] [9]. The detergent micelles used to solubilize them often provide a poor mimic of the native membrane, lacking its specific chemical and physical properties, which can lead to irreversible inactivation [8] [10] [11].
FAQ 2: What is the primary mechanism behind detergent-induced instability?
The instability mechanism often involves the disruption of vital protein-lipid interactions. When extracted with detergents, structure-stabilizing annular lipids can be stripped away from the protein surface [7]. Furthermore, the detergent micelle itself may not adequately accommodate the protein's transmembrane domains, leading to conformational stress and eventual inactivation, as seen in studies of diacylglycerol kinase [9].
FAQ 3: How does the lipid composition of the membrane influence protein stability?
Lipids regulate membrane proteins through a dynamic process called preferential lipid solvation [11]. The protein's surface, which can be geometrically and chemically irregular, is solvated by a fluid layer of lipid molecules. The lipid composition determines the solvation energetics for different protein conformational states. Even minor changes in lipid composition can shift the conformational equilibrium of a protein, thereby influencing its stability and functional activity [11].
FAQ 4: What are the key amino acid factors that influence membrane protein stability?
Stability is influenced by interactions between transmembrane domains. Strategic point mutations within these domains can significantly improve stability [8] [9]. Statistical analyses show that extremostable proteins often have a higher abundance of small nonpolar amino acids like Gly, Val, and Ala in their core, promoting tight packing [12]. Additionally, a significant number of salt bridges on the protein surface can contribute to stability under extreme conditions [12].
Troubleshooting Guides: From Expression to Purification
Troubleshooting Low Expression Yields
Problem: Poor expression levels of the target membrane protein.
Solutions:
- Change Expression Host: Avoid standard BL21(DE3) E. coli for toxic proteins. Use specialized strains like C41(DE3), C43(DE3), or Lemo21(DE3) that reduce transcription rates or allow tunable expression, improving cell health and yield [5] [13].
- Use a Minimal Growth Medium: Counterintuitively, using non-rich media like M9 minimal medium can reduce the cell growth rate, minimizing peptide folding errors in the membrane and potentially improving yields [5].
- Express Homologs: If your target protein does not express well, consider expressing a homologous gene from another species. Subtle differences in the primary sequence can lead to significant improvements in protein stability and expression [5].
- Control Basal Expression: For T7-based systems, use host strains that co-express T7 lysozyme (e.g., pLysS or lysY strains) to inhibit T7 RNA polymerase and prevent toxic basal expression before induction [13].
Troubleshooting Instability During Extraction and Solubilization
Problem: The protein loses activity or aggregates upon extraction from the membrane.
Solutions:
- Systematic Detergent Screening: Identify optimal detergents using high-throughput screens. Classify detergents as "mild" (e.g., non-ionic) or "harsh" (e.g., ionic) and screen for those that maintain stability and functionality [8]. The detergent should be present at a concentration approximately 100 times its Critical Micelle Concentration (CMC) [5].
- Use Lipid-Based Mimics: Replace or supplement detergents with systems that better preserve the native lipid environment:
- Nanodiscs: Engulf membrane sections with native lipids using membrane scaffold proteins [5] [10].
- Peptidiscs: Utilize a short amphipathic bi-helical peptide (NSPr) to wrap around and shield the membrane-exposed part of the protein without requiring additional lipids [10].
- Amphipols: Use polymeric amphiphiles that can stabilize membrane proteins in detergent-free solutions [10].
- Optimize Extraction Conditions: Allow ample time for extraction (3 hours to overnight) and perform it at a slightly elevated temperature (20–30°C) to increase efficiency, provided it does not harm the sample [5].
- Add Stabilizing Ligands: Include substrates, inhibitors, or agonists during purification. Antibodies or nanobodies can also be used to bind and stabilize specific conformational states [8].
Troubleshooting Protein Loss During Purification
Problem: The protein does not bind to affinity columns or is lost during further purification.
Solutions:
- Use Loose Resin: For nickel-affinity chromatography, use loose resin that can be physically mixed with the sample for several hours to encourage binding, as affinity tags can be hidden by large solubilizing agents [5].
- Dilute the Solubilizing Agent: Dilute your sample at least 2-fold to reduce the crowding caused by detergents or other solubilizing agents, giving the affinity tag better access to the resin [5].
- Adjust the Affinity Tag: If binding remains poor, consider moving the affinity tag to the opposite terminus of the protein or lengthening it (e.g., from 6xHis to 12xHis) to push it away from the protein surface [5].
- Improve Purity with Cobalt Resin: For impure samples, charge your affinity resin with cobalt instead of nickel. Cobalt has fewer oxidation states and can increase purity, albeit sometimes at the cost of sample recovery [5].
Quantitative Data and Reagent Solutions
Membrane Protein Stabilization Agents
Table 1: Key Reagent Solutions for Membrane Protein Stabilization
| Reagent / Method | Key Function | Advantages & Considerations |
|---|---|---|
| Detergents (e.g., DDM) | Solubilizes membrane proteins by forming micelles. | Essential for initial extraction; can be destabilizing. Requires screening [8] [5]. |
| Nanodiscs | Stabilizes proteins in a patch of native lipid bilayer surrounded by scaffold proteins. | Preserves native environment; good for functional assays. Can be large and complex to assemble [5] [10]. |
| Peptidisc (NSPr peptide) | Short amphipathic peptide wraps around the transmembrane domain. | "One-size-fits-all", detergent-free, rapid, no added lipids required [10]. |
| Amphipols | Polymeric amphiphiles that stabilize proteins in aqueous solution. | Detergent-free alternative. Can be useful for specific downstream applications [10]. |
| Stabilizing Mutations | Point mutations in transmembrane domains to enhance stability. | Can be identified via alanine scanning or consensus mutagenesis. Requires screening [8] [9]. |
| Ligands / Nanobodies | Bind to and stabilize specific functional conformations of the protein. | Can confer high stability and conformational homogeneity [8]. |
Stoichiometry of the Peptidisc Stabilization Method
Table 2: Quantitative Analysis of the MalFGK2 Peptidisc Complex
| Component | Stoichiometry per MalFGK2 Complex | Method of Determination |
|---|---|---|
| NSPr Peptide | 10 ± 2 peptides | SDS-PAGE [10] |
| Annular Lipids | 41 ± 10 lipids | Thin Layer Chromatography (TLC) [10] |
| Total Mass | 251 ± 12 kDa | Calculation from above [10] |
| Total Mass | 247 ± 24 kDa | Native Mass Spectrometry [10] |
| Total Mass | 250 ± 17 kDa | SEC-MALS [10] |
Experimental Protocols for Stability Assessment
Protocol: High-Throughput Detergent Screening Using FSEC
Purpose: To rapidly identify the optimal detergent for solubilizing and stabilizing a membrane protein.
Method:
- Create Construct: Express the target membrane protein as a fusion with a C-terminal GFP tag [8] [5].
- Solubilize: In a high-throughput format (e.g., 96-well plate), solubilize membranes containing the fusion protein with a library of different detergents.
- Clarify: Centrifuge the solubilized mixtures to remove insoluble material.
- Analyze by FSEC: Inject the supernatant onto a size-exclusion chromatography (SEC) column coupled with a fluorescence detector (excited by GFP fluorescence).
- Evaluate: A sharp, symmetric peak indicates a monodisperse, stable protein. Broad or multiple peaks suggest aggregation or heterogeneity [8] [5].
Protocol: Thermostability Assay for Mutant Screening
Purpose: To screen libraries of mutant membrane proteins for variants with enhanced stability.
Method:
- Create Mutant Library: Generate a library of mutants via random or site-directed mutagenesis [8] [9].
- Express and Solubilize: Express the mutants, pellet the cells, and solubilize the membranes in a chosen detergent.
- Heat Challenge: Aliquot the solubilized samples. Incubate one aliquot at an elevated temperature for a set time, while keeping a control aliquot on ice.
- Assay Activity: Measure the remaining functional activity (e.g., enzymatic activity, ligand binding) in both heated and control samples.
- Identify Stabilizing Mutants: Clones that retain a higher percentage of activity after heating are considered more stable and are selected for further study [9].
Stability Mechanisms and Workflow Visualizations
Membrane Protein Instability Pathway
Membrane Protein Stabilization Workflow
Why is Quality Assessment for Membrane Proteins Crucial?
Membrane proteins are fundamental to cellular life, acting as gatekeepers, signal transducers, and molecular transporters. They represent over 60% of current drug targets, making their study paramount in biomedical research and drug development [14] [15]. However, their inherent instability outside the native lipid bilayer environment makes quality assessment a critical, non-negotiable step in any experimental workflow. Unlike soluble proteins, membrane proteins require a multifaceted approach to quality control that confirms not just purity, but also structural integrity and function [16] [15]. A preparation of high quality is one that is pure, monodisperse, in its native conformation, and functionally active.
Core Quality Metrics and Their Assessment
A robust quality control pipeline for membrane proteins relies on several interconnected metrics. The table below summarizes the key parameters and the primary methods used to evaluate them.
Table 1: Essential Quality Metrics for Membrane Protein Preparations
| Quality Metric | Description | Primary Assessment Methods |
|---|---|---|
| Purity | The proportion of the target protein in the sample relative to contaminants. | SDS-PAGE, Size-Exclusion Chromatography (SEC), Mass Spectrometry [5] [17] |
| Monodispersity & Homogeneity | The uniform distribution of a single protein species in solution, without aggregation. | Size-Exclusion Chromatography (SEC), Dynamic Light Scattering (DLS), Analytical Ultracentrifugation [5] |
| Native Conformation & Oligomeric State | The correct folding and assembly of the protein, including its quaternary structure. | Native Mass Spectrometry (nMS), Size-Exclusion Chromatography with Multi-Angle Light Scattering (SEC-MALS), X-ray Crystallography, Cryo-EM [17] [16] |
| Functional Integrity | The protein's ability to perform its biological activity (e.g., bind ligands, transport ions). | Surface Plasmon Resonance (SPR), Thermal Shift Assays (TSA), Enzymatic or Transport Assays [15] |
| Lipid/Detergent Environment | The composition and properties of the membrane mimetic surrounding the protein. | Native Mass Spectrometry (nMS), Fluorescence Spectroscopy [17] [11] |
Troubleshooting Common Issues in Membrane Protein Preparation
FAQ 1: My membrane protein is not expressing, or the yield is very low. What can I do?
Possible Causes and Solutions:
- Toxicity to Host Cells: Membrane protein overexpression can be toxic to standard expression systems like E. coli BL21.
- Solution: Switch to specialized competent cell strains such as C41(DE3) or C43(DE3), which have mutations that reduce protein expression rates, making them more tolerant of toxic membrane proteins [5].
- Poor Folding in the Membrane:
- Solution: Use a minimal growth medium (e.g., M9), which can slow the cell growth rate and reduce peptide folding errors in the membrane [5].
- Inefficient Extraction from the Membrane: The choice of solubilizing agent is critical.
- Solution: Optimize the detergent used for extraction. Ensure it is present at a concentration of approximately 100x its Critical Micelle Concentration (CMC). Allow sufficient time for extraction (3 hours to overnight) at a slightly elevated temperature (20-30°C) to increase efficiency [5].
FAQ 2: My protein is aggregating during purification. How can I improve monodispersity?
Possible Causes and Solutions:
- Loss of Native Lipid Environment: Removing the protein from its native membrane can expose hydrophobic surfaces, leading to aggregation.
- Solution: Use membrane mimetics that better preserve the native lipid environment. Consider switching from traditional detergents to nanodiscs or amphipols, which can engulf entire sections of the cell membrane along with the protein, preserving its native oligomerization state and preventing aggregation [5] [17].
- Harsh Buffer Conditions:
- Solution: Optimize buffer conditions by including stabilizing additives such as glycerol, lipids, or reducing agents to prevent degradation and aggregation [18].
- Sample Heterogeneity: Heterogeneous samples can lead to broad peaks in chromatography and poor results in downstream applications.
- Solution: Implement additional chromatography steps, such as ion exchange, or optimize existing ones. For affinity purification with a His-tag, using a loose resin and mixing for several hours can improve binding. Diluting the sample to reduce the concentration of the solubilizing agent can also help the affinity tag access the resin [5] [17].
FAQ 3: How can I confirm my purified membrane protein is correctly folded and functional?
Possible Causes and Solutions:
- Lack of Functional Assays:
- Solution: Implement biophysical and functional assays.
- Thermal Shift Assays (TSA) can probe stability by measuring the protein's melting temperature (( T_m )), which often increases when a ligand is bound [15].
- Surface Plasmon Resonance (SPR) can directly measure real-time binding kinetics with ligands or other proteins, confirming functional integrity [15].
- Native Mass Spectrometry (nMS) is a powerful tool for determining the intact molecular mass, oligomeric state, and even identifying bound lipids or small molecules under non-denaturing conditions [17].
- Solution: Implement biophysical and functional assays.
- Incorrect Oligomeric State:
- Solution: Use Size-Exclusion Chromatography with Multi-Angle Light Scattering (SEC-MALS) to accurately determine the absolute molecular weight and oligomeric state in solution. Note that the hydrodynamic radius of a membrane protein in detergent is not a reliable indicator of its molecular weight due to the large, variably shaped detergent micelle [5].
FAQ 4: My purification resin isn't binding my protein effectively. What's wrong?
Possible Causes and Solutions:
- Affinity Tag is Inaccessible: The solubilizing detergent or the protein's own structure can hide the affinity tag.
- Solution: Dilute your sample at least 2-fold to reduce the crowding effect of the solubilizing agent. If using a His-tag, consider lengthening the tag (e.g., from 6xHis to 12xHis) or moving it to the opposite terminus of the protein [5].
- Solution: For nickel-affinity chromatography, use a loose resin that can be physically mixed with the sample for several hours rather than a static column, allowing better access to the tag [5].
Essential Experimental Protocols
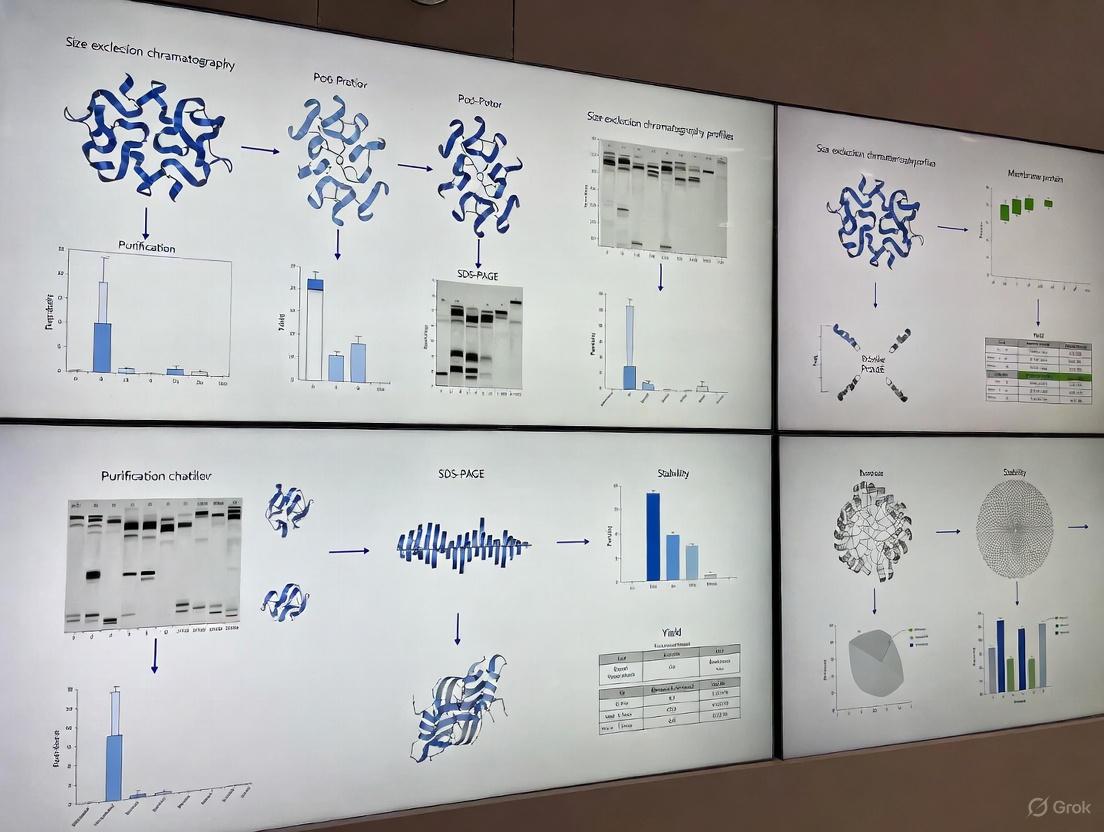
Protocol 1: Assessing Monodispersity and Oligomeric State by Size-Exclusion Chromatography (SEC)
- Column Equilibration: Equilibrate your SEC column with at least two column volumes of your purification or storage buffer, ensuring the detergent is present above its CMC.
- Sample Preparation: Centrifuge your protein sample at high speed (e.g., 15,000 x g) for 10 minutes to remove any insoluble aggregates.
- Sample Injection: Inject a concentrated, small volume of your protein (typically 50-500 µL) onto the column.
- Chromatography: Run the chromatography at a slow, constant flow rate (e.g., 0.5-1.0 mL/min for an analytical column) to ensure proper separation.
- Analysis: Monitor the elution at 280 nm. A sharp, symmetric peak is indicative of a monodisperse sample. A broad or multiple peaks suggest heterogeneity or aggregation [5].
Table 2: Research Reagent Solutions for SEC
| Reagent | Function | Example/Note |
|---|---|---|
| SEC Buffer | Maintains protein stability and detergent micelle integrity during separation. | 20 mM HEPES, pH 7.5, 150 mM NaCl, 0.05% DDM. |
| Detergent | Solubilizes the protein and prevents aggregation. | DDM, LMNG; keep above CMC [5]. |
| SEC Column | Separates protein complexes based on hydrodynamic radius. | Superdex 200 Increase, ENrich 650. |
Protocol 2: Evaluating Thermal Stability by Thermal Shift Assay (TSA)
- Sample Setup: In a real-time PCR tube, mix your membrane protein with a fluorescent dye (e.g., SYPRO Orange, CPM) that binds to exposed hydrophobic patches.
- Thermal Ramp: Place the tube in a real-time PCR instrument and gradually increase the temperature (e.g., from 25°C to 95°C at a rate of 1°C/min).
- Fluorescence Monitoring: The instrument monitors the fluorescence signal. As the protein unfolds, hydrophobic regions are exposed, allowing the dye to bind and increasing fluorescence.
- Data Analysis: Plot fluorescence against temperature. The midpoint of the transition curve (( Tm )) is the melting temperature. A higher ( Tm ) indicates a more stable protein. Ligand binding often stabilizes the protein, resulting in a shift to a higher ( T_m ) [15].
Protocol 3: Confirming Native Conformation and Ligand Binding via Native Mass Spectrometry (nMS)
- Sample Preparation: The membrane protein must be in a volatile buffer compatible with MS (e.g., ammonium acetate) and solubilized in a detergent that facilitates gentle ionization, such as fluorinated surfactants or amphipols [17].
- Ionization: The sample is introduced into the mass spectrometer via Electrospray Ionization (ESI), which gently transfers the protein from solution to the gas phase while preserving non-covalent interactions [17].
- Mass Analysis: The mass-to-charge (m/z) ratios of the ions are determined using high-resolution mass analyzers like Time-of-Flight (TOF) or Orbitrap systems [17].
- Data Interpretation: The resulting spectrum allows for the direct determination of the protein's oligomeric state and molecular mass. The binding of lipids, drugs, or other ligands can be observed as a mass increase, confirming functional interactions in a native-like state [17].
Quality Assessment Workflow
The following diagram illustrates the logical workflow for a comprehensive quality assessment of a membrane protein preparation, from initial purification to functional validation.
The Scientist's Toolkit: Key Research Reagents
Table 3: Essential Materials for Membrane Protein Quality Control
| Category | Reagent / Tool | Function |
|---|---|---|
| Membrane Mimetics | DDM (n-Dodecyl-β-D-maltoside), LMNG (Lauryl Maltose Neopentyl Glycol) | Mild detergents for solubilizing and stabilizing membrane proteins [5]. |
| Nanodiscs, Amphipols | Lipid-based systems that provide a more native-like environment than detergents [5] [17]. | |
| Stabilizing Additives | Glycerol, Lipids, Reducing Agents (DTT) | Prevent aggregation, maintain reducing environment, and enhance stability [18]. |
| Chromatography Resins | Nickel-NTA Resin (Loose) | Affinity purification of His-tagged proteins; loose resin allows better tag access [5]. |
| Cobalt-based Resin | Alternative to nickel for higher purity, though with potentially lower yield [5]. | |
| Analytical Tools | SYPRO Orange / CPM Dye | Fluorescent dyes for Thermal Shift Assays to measure protein stability [15]. |
| Bio-Layer Interferometry (BLI) / SPR Chips | Sensors for label-free analysis of binding kinetics and affinity [15]. |
Membrane proteins are essential for numerous cellular processes, including signal transduction, transport, and cell communication. However, their structural and functional integrity is highly dependent on the native lipid membrane environment. Removing these proteins from their natural context often leads to loss of activity and stability, presenting significant challenges for in vitro studies. Understanding lipid-protein interactions is therefore not merely an academic exercise but a fundamental prerequisite for obtaining high-quality, functional membrane protein preparations for research and drug development. This technical support center addresses the most common experimental issues arising from disruptions to the native membrane environment, providing troubleshooting guides and detailed protocols to help researchers maintain protein stability and function throughout their experiments.
Frequently Asked Questions (FAQs) and Troubleshooting Guides
FAQ 1: Why does my membrane protein lose activity after purification?
Possible Causes and Solutions:
Cause: Disruption of the native lipid solvation environment.
- Solution: Incorporate native lipids or lipid analogs during purification. Studies show that lipid solvation enhances protein stability by facilitating residue burial in the protein interior, a phenomenon reminiscent of the lipophobic effect [19]. Consider using nanodiscs or styrene-maleic acid (SMA) copolymers that preserve native lipid annuli instead of detergents alone [20].
Cause: Removal of specific regulatory lipids.
- Solution: Identify and supplement with crucial lipid species. Research on the CLC-ec1 antiporter demonstrates that lipid composition influences dimerization equilibrium not through specific long-lived lipid binding sites, but via preferential lipid solvation, where certain lipids become enriched at the protein interface due to solvation energetics [11]. Even minor changes in lipid composition (e.g., adding <1% short-chain lipids) can profoundly impact thermodynamic stability [11].
Cause: Destabilization of the cooperative residue-interaction network.
- Solution: Optimize the hydrophobic thickness of your membrane-mimetic system. The lipid bilayer strengthens the cooperative network of membrane proteins. Using amphiphilic assemblies that match the native membrane's hydrophobic thickness and packing strength is critical for stability [19].
FAQ 2: How does lipid composition specifically affect my membrane protein's function?
Lipid composition can alter membrane protein function through multiple, distinct mechanisms, as summarized in the table below.
Table 1: Mechanisms of Lipid Regulation of Membrane Proteins
| Mechanism | Description | Experimental Evidence |
|---|---|---|
| Preferential Lipid Solvation | Dynamic enrichment of specific lipid types at the protein surface alters the conformational equilibrium based on solvation energetics, without long-lived binding [11]. | CLC-ec1 dimerization is inhibited by short-chain DL lipids even at <1% concentration; no saturation effect is observed, ruling out classic binding [11]. |
| Global Bilayer Properties | Changes in bulk membrane properties (thickness, lateral pressure, curvature) couple to protein conformational changes [21]. | Gramicidin A channel lifetime and conductance report on changes in bilayer properties induced by amphitropic proteins like tubulin [21]. |
| Lipid-Mediated Cooperativity | Lipid solvation enhances internal residue interactions, strengthening the protein's cooperative network and its response to stimuli [19]. | Both α-helical and β-barrel membrane proteins from E. coli show increased stability and strengthened residue-interaction networks in lipid bilayers compared to detergents [19]. |
| Competitive Protein-Lipid vs. Protein-Protein Interactions | Strong protein-lipid interactions can compete with and disrupt protein-protein interactions essential for function [22]. | Simulations of RGG protein condensation on membranes show that highly charged lipids can dissolve condensates by outcompeting protein-protein interactions [22]. |
FAQ 3: My membrane protein shows abnormal oligomerization in my assay. Could lipids be the cause?
Yes, this is a common issue. The oligomeric state of membrane proteins is particularly sensitive to the lipid environment.
- Investigate Preferential Solvation: As demonstrated with CLC-ec1, the exposed interface of a monomer can create a local membrane defect (e.g., thinning). Lipids that better solvate this defect (e.g., shorter-chain lipids) will become enriched there, altering the dimerization free energy [11]. Monitor your protein's oligomeric state (e.g., via analytical SEC or native PAGE) in membranes of varying lipid composition.
- Check for a Balance of Interactions: If your protein undergoes liquid-liquid phase separation (LLPS) or clustering on membranes, remember that a delicate balance exists. While some protein-lipid interaction promotes membrane binding and condensation, excessively strong protein-lipid interactions can compete with the protein-protein interactions that drive condensation, leading to dispersion [22].
- Protocol: Assessing Oligomerization State in Different Lipid Compositions
- Reconstitute your purified protein into liposomes or nanodiscs of defined composition. For example, compare a neutral PC lipid (e.g., POPC) with a system containing a fraction of anionic lipids (e.g., POPG) or short-chain lipids (e.g., DLPC) [11] [22].
- Incubate the proteoliposomes at the desired temperature (e.g., 20-30°C can be more efficient than 4°C) for a sufficient time to reach equilibrium (≥3 hours, overnight is often better) [5].
- Analyze the oligomeric state using a technique like Size Exclusion Chromatography (SEC) or Fluorescence Cross-Correlation Spectroscopy (FCCS). Note that SEC of membrane proteins requires caution as the detergent/lipid micelle adds significant, variable mass [5].
- Interpret results: A shift in the oligomeric equilibrium (e.g., from dimer to monomer) upon changing lipid composition indicates strong lipid regulation, likely through preferential solvation [11].
FAQ 4: How can I identify which specific lipids are important for my protein's stability?
- Use Native Nanodiscs or SMA Lipid Particles (SMALPs): These technologies allow for the extraction of a membrane protein along with a "shell" of its native surrounding lipids. Subsequent analysis by mass spectrometry can identify the co-purifying lipid species, which are strong candidates for being functionally important [20].
- Employ Computational Analysis: Molecular dynamics (MD) simulations, particularly coarse-grained MD (CGMD), can predict lipid interactions and enrichment around your protein. This method was used to reveal the sequestration of short-chain DL lipids at the dimerization interface of CLC-ec1 monomers [11].
- Perform Equilibrium Titration Studies: To distinguish between specific lipid binding and weak solvation effects, perform activity or stability assays while systematically titrating in a specific lipid. A lack of saturation in the effect (a linear response) is a key signature of a weak linkage effect like preferential solvation, as opposed to specific, saturable binding [11].
Key Experimental Protocols
Protocol: Quantitative Analysis of Lipid Transport and Its Impact on Protein Environment
Understanding how specific lipid species move between organelles is key to comprehending the dynamic lipid environment of membrane proteins. The following workflow, based on a recent quantitative imaging study, allows for mapping lipid flux [23].
Title: Lipid Transport and Metabolism Analysis Workflow
Detailed Methodology:
- Probe Loading: Load bifunctional lipid probes (containing diazirine and alkyne modifications) into the plasma membrane of live cells (e.g., U2OS) via a brief (0.5-4 min) pulse of α-methyl-cyclodextrin-mediated exchange from donor liposomes. This incorporates lipid molecules into the outer leaflet without compromising PM integrity [23].
- Chase and Metabolism: Remove the loading solution and incubate cells at 37°C for various time points (0 min to 24 h) to allow for intracellular transport and metabolic conversion.
- Crosslinking and Staining: At each time point, photo-crosslink lipids to interacting proteins using UV irradiation, fix cells, and remove non-crosslinked lipids. Fluorescently label the crosslinked bifunctional lipids via click chemistry [23].
- Quantitative Imaging: Acquire confocal images and generate segmented probability maps for organelles (PM, Golgi, ER, endosomes, mitochondria) using pixel classifier software (e.g., Ilastik). Partition the lipid signal between organelles based on these maps [23].
- Mass Spectrometry: In parallel, perform quantitative shotgun lipidomics using ultra-high-resolution Fourier-Transform Mass Spectrometry (FT MS) to track the metabolic conversion of the bifunctional probes at each time point. The mass difference from the diazirine group allows distinction from native lipids [23].
- Kinetic Modeling: Fit the imaging and MS data to a kinetic model that disentangles vesicular and non-vesicular transport routes, yielding rate constants for the interorganelle flux of specific lipid species [23].
Troubleshooting Notes:
- High Background: Ensure thorough removal of non-crosslinked lipids after UV irradiation.
- Poor Signal: Optimize the concentration of bifunctional lipids and the efficiency of the click chemistry reaction.
- This protocol reveals that non-vesicular transport is up to 11 times faster than vesicular transport and is the primary mechanism for fast, species-selective lipid sorting, directly impacting the lipid environment of membrane proteins [23].
Protocol: Discriminating Between Specific Lipid Binding and Preferential Solvation
A critical step in quality assessment is determining the mechanism of lipid regulation. The following protocol outlines a thermodynamic approach to distinguish between these mechanisms [11].
Table 2: Key Characteristics of Lipid Regulation Mechanisms
| Feature | Specific Lipid Binding | Preferential Lipid Solvation |
|---|---|---|
| Conceptual Model | Lipid as a ligand | Lipid as a solvent component |
| Saturation | Yes, follows a binding isotherm | No, effect does not saturate |
| Lifespan of Interaction | Long-lived, specific | Dynamic, transient |
| Key Experimental Test | Titrate lipid and look for saturable effect on protein function/stability. | Titrate lipid and look for non-saturating, linear-like effect on dimerization constant or conformational equilibrium. |
Experimental Steps:
- Reconstitute the membrane protein of interest (e.g., CLC-ec1) into a reference lipid bilayer (e.g., POPC).
- Measure the functional or structural equilibrium parameter of interest (e.g., dimerization constant, activity) in the reference membrane. Use a technique like single-molecule fluorescence or analytical ultracentrifugation.
- Titrate the lipid species under investigation (e.g., DLPC) into the reference membrane at increasing mol% concentrations, starting from very low levels (<1%).
- Measure the functional/structural parameter at each concentration point.
- Analyze the linkage: Plot the change in the equilibrium parameter (e.g., ΔG of dimerization) against the mol% of the titrated lipid.
- A saturating binding curve suggests specific lipid binding at a defined site.
- A non-saturating, near-linear response (observed even at very low concentrations) is characteristic of preferential solvation [11].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents for Studying Lipid-Protein Interactions
| Reagent / Material | Function and Application | Key Considerations |
|---|---|---|
| Nanodiscs / SMALPs | Membrane-mimetic systems that solubilize proteins while preserving a more native-like lipid bilayer environment than detergents. Ideal for functional studies and identifying native co-purifying lipids [20]. | Choose scaffold protein size (for nanodiscs) to match protein diameter. SMALPs extract proteins with native lipids but can have pH/salt limitations. |
| Bifunctional Lipid Probes | Minimally modified lipids (e.g., with diazirine and alkyne) used to track lipid transport and localization in cells via fluorescence microscopy and MS [23]. | Confirm that modifications do not alter the lipid's native membrane properties (e.g., phase behavior). |
| Coarse-Grained (CG) Force Fields (e.g., Martini) | Enables microsecond-to-millisecond timescale Molecular Dynamics (MD) simulations of proteins in complex lipid membranes to study lipid dynamics and enrichment [11] [22]. | Requires conversion from all-atom structures. Martini 3 offers improved accuracy. Simulation timescales are accelerated compared to all-atom. |
| C41(DE3) / C43(DE3) E. coli Cells | Bacterial expression strains with mutated promoters for gentler, slower expression of membrane proteins, reducing toxicity and improving yields of functional protein [5]. | Superior to standard BL21(DE3) for toxic membrane proteins. Lemo21(DE3) is another option for fine-tuning expression. |
| Loose Affinity Resin (e.g., Ni-NTA) | For purifying His-tagged membrane proteins. Loose resin allows for constant mixing, improving access of the often-buried affinity tag to the resin. | Static columns often yield poor binding. If loose resin is unavailable, use a peristaltic pump for closed-loop recirculation over the column [5]. |
| Gramicidin A (grA) | A small channel-forming peptide used as a sensitive reporter of its lipid environment. Changes in grA channel lifetime and conductance report on global and local membrane properties, respectively [21]. | Ideal for studying how amphitropic proteins or other perturbations alter bilayer properties (elastic modulus, curvature, charge). |
The Assessment Toolkit: Biophysical and Functional Methods for Profiling Membrane Protein Preparations
Integral membrane proteins (IMPs) are crucial therapeutic targets, representing nearly two-thirds of all druggable targets due to their roles in signal transduction, cell recognition, and transport processes. However, their inherent hydrophobicity and complex lipid interactions present significant challenges for structural and functional studies. The fundamental step in these investigations involves extracting proteins from their native lipid environment and stabilizing them in aqueous solution using membrane mimetics. These mimetics range from traditional detergents to advanced detergent-free systems that better preserve native protein structure and function. Selecting the appropriate mimetic is therefore critical for successful outcomes in downstream applications such as structural biology, biophysical characterization, and drug discovery. This guide provides a systematic framework for this selection process and troubleshooting common experimental hurdles.
Research Reagent Solutions: A Toolkit for Membrane Protein Studies
The following table summarizes key reagents used in membrane protein solubilization and stabilization, highlighting their primary functions and characteristics.
Table 1: Essential Research Reagents for Membrane Protein Studies
| Reagent Category | Specific Examples | Primary Function | Key Characteristics |
|---|---|---|---|
| Conventional Detergents | DDM, LMNG, GDN, BOG [24] | Solubilize proteins by forming micelles around hydrophobic regions | Mild detergents (e.g., DDM) preserve stability; harsher ones (e.g., SDS) cause denaturation [24] |
| Membrane Scaffold Proteins (MSPs) | ApoA-I-based MSPs [25] [24] | Form Nanodiscs by encircling a lipid bilayer patch | Provides a more native lipid environment; requires detergent for initial extraction [24] |
| Amphipathic Polymers | SMA, DIBMA [26] [24] | Directly solubilize membranes to form native Nanodiscs (e.g., SMALPs) | Detergent-free extraction; preserves native lipids and local membrane environment [26] |
| Amphipathic Peptides | Peptidisc, DeFrMSPs [27] [25] | Stabilize membrane proteins in water-soluble complexes | "One-size-fits-all" property; compatible with mass spectrometry; enables detergent-free workflows [27] |
| Computational Design Tools | AF2seq, ProteinMPNN [28] | Design soluble analogues of membrane protein folds | Creates stable, soluble versions of complex membrane topologies like GPCRs [28] |
Troubleshooting Guide: FAQs and Solutions
FAQ 1: My membrane protein loses activity after detergent solubilization. What are my options?
Issue: Detergents can strip essential lipids or disrupt protein complexes, leading to loss of function [25] [24].
Solutions:
- Switch to a Milder Detergent: If using a harsh detergent, switch to milder alternatives like Lauryl Maltose Neopentyl Glycol (LMNG) or Glycodiosgenin (GDN), which are known to better preserve the stability and function of many membrane proteins [24].
- Use a Detergent-Free System: Transition to a polymer- or peptide-based mimetic that retains native lipids.
- SMALP/DIBMA: Styrene-maleic acid (SMA) or diisobutylene-maleic acid (DIBMA) copolymers can directly solubilize proteins and lipids from the membrane into native nanodiscs, preserving the local lipid environment [26] [24].
- Peptidisc/DeFrMSPs: Use amphipathic peptides like Peptidisc or DeFrMSPs for detergent-free reconstitution. This has been shown to maintain functional states of sensitive transporters, such as the MalFGK2 ABC transporter, whose ATPase activity is uncoupled in detergents but remains coupled in peptide-based nanodiscs [27] [25].
FAQ 2: My protein is solubilized but aggregates during purification or on the Cryo-EM grid. How can I prevent this?
Issue: Aggregation can occur due to protein instability, exposure of hydrophobic surfaces, or unsuitable buffer conditions.
Solutions:
- Screen Scaffolding Agents: Incorporate a screening step with different scaffolding agents to find the optimal one for your protein.
- Peptide Screening: As demonstrated with DeFrMSPs, screen a panel of amphipathic peptides with different modifications (e.g., fatty acid conjugations) to find the condition that yields monodisperse particles [25].
- Polymer Screening: A large library of different polymers may be required for optimal ND reconstitution, as their performance can be protein-specific [25].
- Optimize the Lipid Environment: Use nanodisc systems (MSP-based or polymer-based) that provide a lipid bilayer shield, which can protect hydrophobic surfaces and prevent aggregation [24].
- Assess Sample Quality Early: Implement Fluorescent Size Exclusion Chromatography (FSEC) to rapidly analyze sample quality and monodispersity using only small quantities of sample before committing to large-scale purification [29].
FAQ 3: My target is a large or dynamic membrane protein complex that falls apart in detergents. What strategies can I use?
Issue: Large, multi-subunit complexes are often destabilized by detergents, which can dissociate subunits.
Solutions:
- Bypass Detergents Entirely: Employ a direct, detergent-free extraction method.
- DeFrND Protocol: Use the DeFrND (detergent-free reconstitution into native nanodiscs) method with engineered membrane scaffold peptides (DeFrMSPs) to directly pull the intact complex from the native membrane, preserving its stoichiometry and integrity [25].
- Consider a Computational Approach: For some applications, consider the novel approach of computationally designing a soluble analogue of your membrane protein target. Deep learning pipelines (e.g., AF2seq-MPNN) can now design stable, soluble proteins that recapitulate the complex topologies of IMPs like GPCRs and rhomboid proteases, potentially bypassing handling challenges altogether [28].
FAQ 4: How can I systematically select the best detergent or mimetic for a new membrane protein?
Issue: The optimal mimetic is highly protein-specific, and a rational screening approach is needed.
Solution: Implement a tiered screening workflow.
Diagram 1: A tiered mimetic screening workflow for systematic optimization.
Experimental Protocol for a Mimetic Screen:
- Solubilization Test: Create small-scale aliquots of membrane preparation. Solubilize each with a different detergent (e.g., DDM, LMNG) or polymer (e.g., SMA) at a concentration slightly above its CMC for 1-2 hours on ice.
- Ultracentrifugation: Centrifuge at high speed (e.g., 100,000 × g) to separate solubilized protein (supernatant) from insoluble material (pellet).
- Analysis: Analyze the supernatant and pellet by SDS-PAGE to determine solubilization efficiency.
- Stability Assessment: For successful solubilizing conditions, proceed with FSEC [29] to check for aggregation and a functional assay to confirm activity is retained.
FAQ 5: How do I stabilize a membrane protein for Thermal Shift Assay or Thermal Proteome Profiling (TPP)?
Issue: Standard TPP protocols are often incompatible with detergents and have poor coverage of the membrane proteome [27].
Solution: Adopt a detergent-free membrane mimetic strategy specifically designed for stability assays.
Protocol: Membrane-Mimetic Thermal Proteome Profiling (MM-TPP) [27]
- Prepare a Membrane Proteome Library: Solubilize a membrane fraction with a mild detergent and then reconstitute it into a Peptidisc library. This replaces the detergent and stabilizes the membrane proteome in a water-soluble, native-like state.
- Ligand Treatment: Divide the Peptidisc library into two aliquots. Incubate one with the ligand of interest and the other with a vehicle control (e.g., ddH₂O).
- Heat Denaturation: Subject the samples to a range of elevated temperatures (e.g., 3 minutes at each temperature) to induce protein denaturation and precipitation.
- Separation and Analysis: Remove precipitated protein by ultracentrifugation. Analyze the soluble fraction (containing thermally stabilized proteins) by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
- Data Analysis: Identify proteins that show a significant shift in thermal stability in the ligand-treated sample compared to the control, as these are high-probability ligand binders.
Diagram 2: The MM-TPP workflow for profiling membrane protein-ligand interactions.
Comparative Analysis of Membrane Mimetic Technologies
The table below provides a structured comparison of the primary mimetic technologies to guide the selection process.
Table 2: Comparison of Membrane Mimetic Technologies
| Technology | Mechanism of Action | Pros | Cons | Best For |
|---|---|---|---|---|
| Traditional Detergents (e.g., DDM, LMNG) | Forms micelles around protein transmembrane domains [24] | Well-established, wide commercial availability, easy to use [24] | Can denature proteins, strip native lipids, disrupt protein complexes [25] [24] | Initial solubilization, proteins stable in micelles |
| MSP Nanodiscs | Membrane scaffold protein encircles a lipid bilayer patch [24] | Provides a more native lipid environment, high stability [24] | Requires detergent for initial extraction, costly, requires optimization [24] | Biophysical studies, functional assays requiring a lipid bilayer |
| Polymer-Based Native Nanodiscs (e.g., SMALP) | Amphipathic polymer directly solubilizes membrane patches [26] [24] | Detergent-free, preserves native lipids and complex integrity [24] | Sensitivity to divalent cations, may require polymer screening, limited Cryo-EM use [25] [24] | Studying proteins in a near-native lipid environment, detergent-sensitive complexes |
| Peptide-Based Systems (e.g., Peptidisc, DeFrND) | Amphipathic peptides form a protective belt around the protein [27] [25] | Detergent-free, "one-size-fits-all" stability, MS-compatible, can maintain functional coupling [27] [25] | Peptide optimization may be needed for some targets, relatively new technology | Thermal shift assays (MM-TPP), functional studies, proteome-wide screens [27] |
| Computational Soluble Analogues | Deep-learning design of soluble versions of IMP folds [28] | Completely bypasses membrane handling, high stability [28] | Not the native protein, may not capture all functional aspects, cutting-edge method [28] | Drug discovery on novel epitopes, fundamental protein design research [28] |
Analytical Ultracentrifugation (AUC) is a first-principles, solution-based technique that plays a critical role in the biophysical characterization of macromolecules, including challenging membrane protein preparations. For researchers investigating membrane protein quality, AUC provides unmatched capabilities for determining molecular weight, assessing sample purity, and quantifying states of aggregation—all without requiring a solid matrix or labeling that could disrupt the native state of the protein. Unlike techniques such as size exclusion chromatography (SEC) that involve stationary phases and potential disruptive interactions, AUC allows membrane proteins to be analyzed in their true solution environment, even in the presence of detergents and lipids necessary for stability [30] [31]. This technical support center provides comprehensive guidance for implementing AUC in your membrane protein research, addressing common challenges through detailed protocols, troubleshooting advice, and expert recommendations.
For membrane protein scientists, AUC serves as an essential orthogonal method to validate results obtained from other techniques. Its ability to directly quantify soluble aggregates—a critical quality attribute for therapeutic proteins—makes it invaluable for pre-formulation studies and stability assessments [30]. Furthermore, AUC's broad dynamic range, capable of analyzing particles from kilodaltons to gigadaltons and concentrations from picomolar to millimolar, ensures its relevance across diverse experimental setups from initial membrane protein characterization to advanced interaction studies [31].
Key Principles and Methodologies
Fundamental Concepts
AUC operates on two fundamental physical principles: sedimentation under centrifugal force and the relationship between sedimentation behavior and macromolecular properties as described by the Svedberg equation:
[ s = \frac{M(1-\overline{v}ρ)}{N_A f} ]
where (s) is the sedimentation coefficient, (M) is molecular weight, (\overline{v}) is the partial specific volume, (ρ) is solvent density, (N_A) is Avogadro's number, and (f) is the frictional coefficient [31]. This equation establishes the direct relationship between observable sedimentation and molecular properties, allowing researchers to extract accurate molecular parameters without external standards.
Operational Modes
AUC offers two primary operational modes, each with distinct advantages for membrane protein characterization:
Sedimentation Velocity (SV-AUC) applies high centrifugal forces to observe the rate at which molecules migrate through solution. This mode is particularly valuable for assessing sample heterogeneity, detecting aggregates, studying conformational changes, and determining hydrodynamic properties [32] [33]. The sedimentation coefficient (measured in Svedbergs, S) provides information about both molecular size and shape.
Sedimentation Equilibrium (SE-AUC) operates at lower speeds where sedimentation and diffusion forces balance, creating a stable concentration gradient. This method enables precise determination of absolute molecular weight, study of reversible interactions, and thermodynamic characterization of self-associating systems [32] [33].
Table: Comparison of AUC Operational Modes for Membrane Protein Research
| Parameter | Sedimentation Velocity (SV-AUC) | Sedimentation Equilibrium (SE-AUC) |
|---|---|---|
| Primary Applications | Size distribution analysis, aggregate detection, sample heterogeneity, conformational studies | Molecular weight determination, binding constants, stoichiometry of interactions |
| Experimental Speed | High speeds (40,000-60,000 rpm) | Lower speeds (10,000-25,000 rpm) |
| Experimental Duration | Several hours | Several hours to days |
| Data Output | Sedimentation coefficient distribution | Molecular weight and association constants |
| Information Content | Hydrodynamic properties, sample purity, aggregation state | Thermodynamic parameters, equilibrium constants |
| Ideal for Membrane Protein Studies | Assessing detergent-solubilized protein homogeneity, detecting aggregated species | Determining oligomeric state in detergent solutions |
Essential Reagents and Materials
Research Reagent Solutions
Successful AUC experiments require careful preparation and selection of appropriate reagents. The following table outlines essential materials for membrane protein AUC studies:
Table: Essential Research Reagents for Membrane Protein AUC Studies
| Reagent/Material | Function/Purpose | Key Considerations |
|---|---|---|
| Appropriate Buffer System | Maintains protein stability and native conformation | Must be compatible with detergents; should match reference buffer for interference optics [30] |
| Detergents | Solubilizes and stabilizes membrane proteins | Critical for maintaining solubility; choice affects partial specific volume and density calculations [34] |
| Density Modifiers | Adjusts solvent density for optimal sedimentation | Glycerol, sucrose, or D₂O may be used; requires precise measurement of resulting solvent density [30] |
| Reference Buffer | Matches solvent environment for interference optics | Must be dialyzed against sample buffer when using interference detection [33] |
| Absorbance Standards | Verifies optical system performance | Needed for quantitative concentration measurements at specific wavelengths |
Experimental Design and Protocols
Sample Preparation Protocol
Proper sample preparation is crucial for obtaining reliable AUC data, particularly for sensitive membrane protein systems:
Sample Purity and Characterization: Begin with the purest membrane protein preparation achievable. Prior characterization using complementary methods such as fluorescent size exclusion chromatography (FSEC) can provide initial quality assessment and save valuable AUC instrument time [35] [29].
Buffer Matching and Dialysis: For interference detection, carefully dialyze the membrane protein sample against the reference buffer to minimize signal from buffer mismatches [30] [33]. For absorbance detection, SEC buffer exchange may be sufficient.
Concentration Optimization: Target an absorbance of approximately 1.0 for absorbance-based detection to ensure optimal signal-to-noise ratio while maintaining linear detector response [30]. For membrane proteins with low extinction coefficients, consider using interference detection.
Density and Viscosity Measurements: Precisely measure or calculate solvent density and viscosity, as these parameters significantly impact sedimentation coefficient calculations. This is particularly important for detergent-containing buffers, which may have different physical properties than aqueous solutions.
Control for Co-sedimenting Solutes: When formulating with sugars or polyols that may form density gradients during centrifugation, include control experiments in sugar-free buffers or apply appropriate inhomogeneous solvent models during data analysis [30].
Basic SV-AUC Experimental Workflow
The following diagram illustrates the key steps in a sedimentation velocity experiment for membrane protein characterization:
Basic SV-AUC Experimental Workflow
Data Collection Parameters
For membrane protein studies using SV-AUC, implement the following data collection protocol:
Rotor Selection: Use an An-Ti50 rotor or equivalent suitable for the required speeds and sample volumes.
Temperature Control: Set temperature to 20°C for standard experiments, or adjust based on membrane protein stability requirements.
Speed Selection: Program rotor speed between 40,000-60,000 rpm depending on the expected size of the membrane protein complex and detergent micelle.
Data Acquisition: Collect absorbance (230-280 nm) and/or interference data at 2-3 minute intervals throughout the run duration.
Radial Resolution: Set radial step size to 0.003 cm for high-resolution data collection.
Run Duration: Continue centrifugation until complete clearance of the meniscus is observed for all sedimenting species.
Troubleshooting Common AUC Challenges
Frequently Asked Questions
Q1: Our membrane protein preparation shows significant aggregation in SV-AUC but not in SEC. Which result should we trust?
A1: SV-AUC is generally more reliable for aggregate detection since it occurs in solution without a stationary phase that can potentially trap or dissociate aggregates [30]. SEC can sometimes dissociate weakly associated aggregates through dilution effects or interactions with the column matrix. The absence of a solid matrix in AUC makes it less prone to such artifacts. We recommend trusting the AUC results and investigating buffer conditions that might improve monodispersity.
Q2: How do we account for the contribution of detergent micelles to the sedimentation of our membrane protein?
A2: The detergent contribution presents a common challenge in membrane protein AUC. Several approaches can help:
- Characterize the detergent alone under identical buffer conditions to determine its sedimentation coefficient.
- Consider using contrast matching through density modulation, though this is challenging with conventional detergents.
- Analyze the protein-detergent complex as a single entity and interpret the resulting molecular weight accordingly.
- In some cases, using minimal detergent concentrations while maintaining solubility can reduce this complication.
Q3: Why do we get different aggregation percentages between AUC and SEC methods?
A3: Discrepancies often arise from fundamental methodological differences [30]:
- SEC may dissociate aggregates during separation or through dilution effects.
- AUC measures all material in the sample, while SEC may miss extremely large aggregates that are excluded from column pores.
- Interactions with the SEC stationary phase can retain certain species.
- Always consider AUC as the more accurate measure for solution-state aggregation.
Q4: What are the key considerations for selecting between absorbance and interference detection?
A4: The choice depends on your specific application:
- Use absorbance detection when working with purified membrane proteins at appropriate concentrations (A280 ~0.1-1.0).
- Interference detection is preferable for samples with low extinction coefficients or when using detergents that absorb in the UV range.
- For complex detergent systems, interference detection often provides better signal-to-noise.
- Remember that interference detection requires careful buffer matching through dialysis.
Q5: How can we improve the resolution between monomeric and dimeric species of our membrane protein?
A5: Several strategies can enhance resolution:
- Optimize rotor speed - slightly lower speeds may improve separation of closely sedimenting species.
- Ensure optimal sample concentration to minimize concentration-dependent association.
- Extend data collection time to improve boundary separation.
- Consider using SE-AUC for precise molecular weight determination of each species.
- Explore buffer modifications (pH, salt) that might alter the association equilibrium.
Advanced Applications in Membrane Protein Research
Specialized Methodologies
AUC offers several advanced applications particularly valuable for membrane protein research:
Ligand-Induced Conformational Changes: By comparing sedimentation coefficients in the presence and absence of ligands, researchers can detect ligand-induced conformational changes in membrane proteins. Such changes often alter hydrodynamic properties detectable by SV-AUC [32].
Detergent Optimization Studies: SV-AUC serves as a powerful tool for screening detergents and stabilization conditions for membrane proteins. The method can rapidly identify conditions that minimize aggregation and improve monodispersity [34].
Complex Stoichiometry Determinations: SE-AUC can accurately determine the stoichiometry of membrane protein complexes, even in mixed detergent-lipid systems, providing critical information about functional assemblies.
Stability Assessment: Through thermal or chemical challenge experiments monitored by AUC, researchers can assess the stability of membrane protein preparations under different solution conditions.
Data Analysis and Interpretation Framework
The following diagram illustrates the decision process for analyzing and interpreting AUC data from membrane protein experiments:
AUC Data Analysis Decision Framework
Analytical Ultracentrifugation remains an indispensable tool in the membrane protein researcher's toolkit, providing critical information about molecular weight, purity, and aggregation state that is difficult to obtain through other methods. Its solution-based, matrix-free nature makes it particularly valuable for studying delicate membrane protein systems that may be affected by surfaces or matrices in other techniques.
As the field advances, developments in AUC instrumentation and software are moving the technique toward compliance with Good Manufacturing Practices (GMP) environments, expanding its utility from basic research to pharmaceutical development and quality control [36]. The implementation of fluorescence detection systems has further extended AUC's capabilities to study increasingly complex systems at lower concentrations [31].
For membrane protein scientists, AUC provides the rigorous hydrodynamic characterization necessary to advance our understanding of structure-function relationships in this biologically crucial class of proteins. By following the protocols and troubleshooting guidance outlined in this technical support document, researchers can leverage the full power of AUC to overcome common challenges in membrane protein characterization and accelerate their research progress.
FAQs: Core Principles and Applications
Q1: What is the key advantage of adding MALS to a standard SEC setup for membrane protein analysis? SEC-MALS determines the absolute molecular mass of a protein complex in solution independently of its elution volume. This is crucial for membrane proteins, which often bind detergent micelles. While SEC alone separates by hydrodynamic size, MALS allows you to distinguish the mass of the protein oligomer from the mass of the associated detergent, providing the true oligomeric state and confirming sample homogeneity [37].
Q2: Why is my light scattering baseline high and noisy, especially after installing a new column? This is a common issue. Light scattering detectors are extremely sensitive to large particles and aggregates, including nanometer-sized particles and fines that can bleed from the column packing material. These contaminants are often too small to be trapped by column frits and can cause a persistently high baseline and noise. This is particularly problematic for low-angle light scattering measurements and aqueous mobile phases [37].
Q3: How can I tell if my SEC-MALS system is clean enough for sensitive analysis? A clean system shows a stable, low-noise baseline on both the concentration detector (RI or UV) and the light scattering detector. If the concentration detector baseline is stable but the light scattering signal shows high background or drift, the system—often the column itself—is likely the source of contamination and requires cleaning or replacement with an LS-certified column [37].
Q4: My membrane protein tends to aggregate. What techniques can I use alongside SEC-MALS to assess homogeneity? SEC-MALS is an excellent primary tool, but homogeneity can be confirmed using orthogonal techniques:
- Dynamic Light Scattering (DLS): Rapidly assesses the size distribution and monodispersity of a sample in solution, directly detecting aggregates [38] [39].
- Analytical Ultracentrifugation (AUC): Provides detailed information on molecular weight, oligomeric state, and conformation through sedimentation behavior [39].
- 05SAR-PAGE: A specialized electrophoresis method effective for resolving membrane protein oligomers and complexes without disrupting weak interactions [40].
Troubleshooting Guide
Table 1: Common SEC-MALS Issues and Solutions
| Symptom | Possible Root Cause | Recommended Solution |
|---|---|---|
| High LS baseline & noise | Contaminants from new column [37] | Flush column extensively per manufacturer's instructions; use columns pre-treated for LS [37] |
| Particulates in mobile phase or sample [37] | Filter all solvents through 0.1 µm filters; centrifuge or filter samples before injection [37] | |
| Broad/Asymmetric Peaks | Column overloading [41] | Reduce injection volume or sample concentration [41] |
| Non-specific interaction with column [42] | Adjust mobile phase (e.g., add salt); use a different column chemistry [42] | |
| Aggregated protein sample | Optimize buffer conditions; include stabilizing additives | |
| Unexpectedly High Molecular Mass | Protein aggregation [39] | Check for homogeneity with DLS; use fresh sample; optimize buffer [38] [39] |
| Low LS Signal | Sample mass or concentration too low [37] | Increase sample concentration; ensure dn/dc value is correct |
| Peak Tailing | Secondary interactions with column [42] | Modify mobile phase pH or ionic strength; use a shield-phase column [42] |
| Pressure Fluctuations/High Pressure | Column blockage [41] [42] | Reverse-flush column if possible; replace guard column; filter samples [41] [42] |
Experimental Protocols
Protocol 1: Assessing Membrane Protein Oligomerization and Homogeneity by SEC-MALS
Objective: To determine the absolute molecular mass and oligomeric state of a purified membrane protein in solution.
Materials:
- Purified membrane protein in a compatible buffer.
- SEC column (e.g., silica- or polymer-based) appropriate for the protein's size.
- SEC-MALS system (comprising HPLC, SEC column, MALS detector, and RI detector).
- Degassed, filtered running buffer (e.g., with detergent to maintain solubility).
Method:
- System Equilibration: Equilibrate the entire SEC-MALS system with the running buffer until stable baselines are achieved on both the RI and LS detectors [37].
- Sample Preparation: Centrifuge the protein sample at high speed (e.g., 15,000 x g) to remove any large aggregates or particles. Use the same running buffer for the sample [37].
- Injection and Separation: Inject the clarified protein sample onto the SEC column. Typical injection volumes range from 10-100 µL, depending on column size.
- Data Collection: The eluent passes sequentially through the MALS detector and then the RI detector. Data is collected for both detectors.
- Data Analysis: Using the ASTRA or similar software:
- The RI detector provides the concentration of the sample.
- The MALS detector measures the scattering intensity at multiple angles.
- The software combines these signals with the dn/dc value (refractive index increment) for the protein-detergent complex to calculate the absolute molecular mass across the entire elution peak.
Interpretation: A homogeneous, monodisperse protein sample will produce a single, symmetric peak with a constant molecular mass across the peak. Fluctuations in mass or multiple peaks indicate heterogeneity, aggregation, or different oligomeric states.
Protocol 2: Confirming Oligomerization by 05SAR-PAGE and Western Blot
Objective: To independently verify the oligomeric state of membrane proteins using a gentle electrophoresis method.
Materials:
- Purified membrane protein sample.
- Gel electrophoresis system.
- 05SAR-PAGE gel (containing 0.05% sarkosyl) [40].
- Transfer system for Western blotting.
- Protein-specific primary antibody and labeled secondary antibody.
Method:
- Sample Preparation: Mix the protein sample with a non-reducing, non-denaturing loading buffer.
- Electrophoresis: Load the sample and run the 05SAR-PAGE gel at constant voltage under cooling conditions to preserve native complexes [40].
- Western Blot: Transfer the separated proteins from the gel to a membrane.
- Detection: Incubate the membrane with a primary antibody specific to your protein, followed by a labeled secondary antibody. Detect the signal.
Interpretation: The apparent molecular weight of the band(s), compared to a standard ladder, indicates the oligomeric state (e.g., monomer, dimer, trimer). A single band suggests homogeneity, while multiple bands suggest multiple states [40].
Visualization of Concepts and Workflows
Diagram 1: SEC-MALS Workflow for Membrane Proteins
Diagram 2: SEC-MALS Data Interpretation Logic
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Materials for Membrane Protein Oligomeric State Analysis
| Reagent / Material | Function in Analysis |
|---|---|
| LS-Certified SEC Columns | Pre-treated packing material minimizes particle fines, reducing background noise in light scattering detectors [37]. |
| Compatible Detergents | Maintains membrane protein solubility and prevents non-specific aggregation during analysis (e.g., DDM, OG). |
| Size-Exclusion Standards | For calibrating column separation range and verifying system performance. |
| dn/dc Value | The refractive index increment; a critical parameter for accurate molecular mass calculation in MALS analysis [37]. |
| Sarkosyl Detergent | Key component of 05SAR-PAGE gels for gentle separation of membrane protein oligomers [40]. |
Frequently Asked Questions (FAQs)
Q1: What is the primary purpose of using antibody accessibility assays in live cells for membrane protein research? Antibody accessibility assays in live cells are crucial for evaluating the native conformation and spatial orientation of membrane proteins. By applying antibodies to non-permeabilized, live cells, researchers can determine which extracellular epitopes are exposed and accessible, providing direct insight into the protein's functional state and structural integrity without artifacts introduced by fixation.
Q2: Why is proper fixation critical for assessing membrane protein conformation, and what are the key considerations? Fixation preserves cellular architecture but can alter membrane protein epitopes through cross-linking, leading to false negatives or misleading results. Over-fixation, in particular, can damage epitopes and mask antibody binding sites [43] [44]. Key considerations include using the correct fixative concentration (e.g., 1-4% formaldehyde), optimizing fixation duration to avoid over-fixation, and using fresh formaldehyde stocks to prevent high autofluorescence [45].
Q3: My assay shows a weak signal despite confirmed protein expression. What are the most likely causes? Weak signal with confirmed expression often stems from technical issues rather than biological ones. The most common causes are:
- Suboptimal Antibody Dilution: The primary antibody may be too dilute [45].
- Insufficient Incubation Time: Primary antibody incubation time may be too short; overnight incubation at 4°C is often recommended for consistent results [45].
- Inadequate Permeabilization (for intracellular targets): The permeabilization method may not allow antibody access to the epitope [45] [43].
- Fluorophore Bleaching: The fluorescent tag may have been degraded by extended light exposure [44].
Q4: How can I minimize high background noise in my live-cell immunofluorescence images? High background is frequently caused by non-specific antibody interactions and can be mitigated by:
- Effective Blocking: Ensure sufficient blocking with normal serum from the secondary antibody host species or charge-based blockers [45] [43].
- Optimized Antibody Concentration: Overly concentrated primary or secondary antibodies are a common cause of background; titrate to find the optimal dilution [45] [44].
- Thorough Washing: Increase wash duration and volume to remove loosely bound antibodies [45] [46].
- Validating Secondary Antibodies: Use a secondary-only control to check for non-specific cross-reactivity [45] [43].
Troubleshooting Guides
Table 1: Troubleshooting Weak or No Signal
| Problem Cause | Recommended Solution | Underlying Principle |
|---|---|---|
| Inadequate Fixation | Follow validated protocols; use at least 4% formaldehyde for phospho-specific antibodies; avoid over-fixation [45] [43]. | Preserves antigenicity while maintaining cell structure. |
| Incorrect Antibody Dilution | Consult manufacturer datasheets for recommended dilution; titrate antibody if necessary [45] [44]. | Ensures optimal antigen-antibody binding kinetics. |
| Insufficient Incubation Time | Increase primary antibody incubation time; validate protocols (e.g., overnight at 4°C) [45]. | Allows for sufficient equilibrium binding for low-abundance targets. |
| Inadequate Permeabilization | Optimize detergent type and concentration (e.g., 0.1-1% Triton X-100); skip if using methanol/acetone [47] [43]. | Enables antibody access to intracellular epitopes without destroying morphology. |
| Fluorophore Bleaching | Perform all incubations in the dark; use anti-fade mounting medium; image immediately [45] [44]. | Prevents photochemical destruction of the fluorophore. |
| Low Protein Expression | Include a positive control (e.g., overexpressing cell line); use signal amplification methods [45]. | Confirms assay sensitivity and validates negative results. |
Table 2: Troubleshooting High Background and Non-Specific Staining
| Problem Cause | Recommended Solution | Underlying Principle |
|---|---|---|
| Insufficient Blocking | Prolong blocking time; use serum from secondary host species or specialized blocking buffers [45] [43]. | Occupies non-specific protein-binding sites on the sample. |
| Antibody Concentration Too High | Reduce concentration of primary and/or secondary antibody; perform a dilution series [45] [44]. | Minimizes off-target, low-affinity binding interactions. |
| Sample Autofluorescence | Use unstained controls; avoid glutaraldehyde; use fresh fixatives; employ longer wavelength channels [45] [43]. | Reduces interference from endogenous fluorescent molecules. |
| Secondary Antibody Cross-reactivity | Run a secondary-only control; pre-spin antibodies to remove aggregates [45] [44]. | Identifies and eliminates non-specific binding of the detection reagent. |
| Insufficient Washing | Increase wash volume, duration, and frequency; ensure complete buffer exchange [45] [46]. | Removes unbound and loosely-associated antibodies. |
| Non-specific Primary Antibody | Compare to knockout controls; use antibodies validated for immunofluorescence [45] [43]. | Confirms the specificity of the antibody-epitope interaction. |
Experimental Protocols
Protocol 1: Live-Cell Antibody Accessibility Assay for Surface Membrane Proteins
This protocol is designed to label and assess the conformation of extracellular domains of membrane proteins in their native, live-cell state.
Key Materials:
- Live cell suspension (viability >90%) [47]
- Ice-cold suspension buffer (e.g., PBS with 5-10% FCS) [47]
- Fluorophore-conjugated primary antibody or isotype control
- Viability dye (e.g., 7-AAD, DAPI) to exclude dead cells [47]
- FcR blocking buffer (e.g., 2-10% goat serum, human IgG, or anti-CD16/CD32 antibodies) [47]
Methodology:
- Sample Preparation: Harvest and wash cells gently in ice-cold suspension buffer. Maintain cells at 0.5–1 x 10⁶ cells/mL to prevent clumping [47].
- Viability Staining: Incubate cells with a viability dye (e.g., 7-AAD) in the dark at 4°C according to the manufacturer's instructions. Wash twice to remove excess dye [47].
- Fc Receptor Blocking: Resuspend the cell pellet in an appropriate FcR blocking buffer. Incubate for 30-60 minutes in the dark at 4°C to prevent non-specific antibody binding [47].
- Surface Staining: Without permeabilizing, incubate cells with the fluorophore-conjugated primary antibody against your target membrane protein. Perform all incubations in the dark on ice to inhibit internalization.
- Washing and Analysis: Wash cells twice thoroughly with ice-cold suspension buffer to remove unbound antibody. Resuspend in buffer for immediate analysis via flow cytometry or live-cell imaging.
Protocol 2: Immunofluorescence for Total Membrane Protein Detection (After Fixation)
This protocol is used to visualize both surface and intracellular pools of a membrane protein, requiring fixation and permeabilization.
Key Materials:
- Fixed cells
- Fixative (e.g., 1-4% Paraformaldehyde (PFA) or 90% Methanol) [47]
- Permeabilization solution (e.g., Triton X-100, Saponin) [47]
- Primary antibody and compatible secondary antibody
- Blocking buffer (e.g., normal serum)
- Mounting medium with anti-fade agent [45]
Methodology:
- Fixation: Aspirate media and wash cells briefly with PBS. Fix cells with your chosen fixative (e.g., 1-4% PFA for 15-20 minutes on ice). Note that methanol also permeabilizes, making a separate step unnecessary [47].
- Permeabilization: If using PFA, incubate cells with a permeabilization detergent (e.g., 0.1% Triton X-100 for 10-15 minutes at room temperature). For cytoplasmic or plasma membrane antigens, mild detergents like saponin may be preferable [47].
- Blocking: Incubate cells with a blocking buffer for at least 30 minutes to reduce background [43].
- Primary Antibody Staining: Incubate with the primary antibody diluted in blocking buffer. Overnight incubation at 4°C is often optimal [45].
- Secondary Antibody Staining: After washing, incubate with a fluorophore-conjugated secondary antibody, matched to the primary antibody host species. Perform all steps in the dark.
- Mounting and Imaging: Mount samples using an anti-fade mounting medium. Image immediately for best results [45].
Research Reagent Solutions
Table 3: Essential Reagents for Membrane Protein Conformation Assays
| Reagent Category | Specific Examples | Function & Importance |
|---|---|---|
| Fixatives | Paraformaldehyde (1-4%), Methanol (90%), Acetone [47] | Preserves cellular structure and immobilizes proteins; choice affects epitope integrity. |
| Permeabilizers | Triton X-100 (harsh), Saponin (mild), Tween-20 [47] | Enables intracellular access by dissolving membranes; harshness must match target. |
| Blocking Agents | Normal Serum, BSA, Commercial Signal Enhancers [45] [47] | Reduces non-specific background by saturating reactive sites. |
| Viability Dyes | 7-AAD, DAPI, TOPRO3, Fixable Amine-Reactive Dyes [47] | Distinguishes live from dead cells; critical for excluding dead cells in live assays. |
| FcR Blockers | Species-Specific IgG, Anti-CD16/CD32 Antibodies [47] | Blocks Fc receptors to prevent false-positive signal from antibody non-specific binding. |
| Anti-fade Mountants | ProLong Gold Antifade Reagent [45] | Retards fluorophore photobleaching during microscopy, preserving signal. |
Experimental Workflow and Quality Control
The following diagram illustrates the critical decision points and pathways for successfully evaluating membrane protein conformation using immunofluorescence and antibody accessibility assays.
For researchers focusing on purified membrane protein systems, techniques like Fluorescent Size Exclusion Chromatography (FSEC) provide a rapid, small-scale method to assess protein quality, monodispersity, and oligomeric state before undertaking more complex cellular assays [29]. Integrating these biophysical quality controls with cellular data strengthens conclusions about native conformation.
FAQs and Troubleshooting Guides
Q1: Our enzymatic hydrolysis assay for a membrane protein shows unexpected sigmoidal kinetics instead of standard Michaelis-Menten hyperbolic kinetics. What could be the cause?
A: Sigmoidal kinetics can arise from the specific interaction between your substrate, its soluble binding protein, and the membrane-bound acceptor protein. The shape of the saturation curve is diagnostically important [48]:
- If the acceptor (your membrane protein) acts on the unbound ligand, performing assays at a constant concentration of binding protein will show progressively increasing inhibition and a shift from near-hyperbolic to sigmoidal curves as the binding affinity of the protein increases [48].
- If the acceptor acts on the protein-bound ligand, the maximum velocity obtained at a constant binding-protein concentration will increase hyperbolically with that concentration [48].
- Check your assay conditions: Ensure the amount of acceptor-bound substrate is small compared to the total amount and that all preceding chemical reactions and mass transport steps are rapid compared to the catalytic step you are monitoring [48].
Q2: We observe a weak or absent signal in our flow cytometry-based functional assay. What are the most common causes and solutions?
A: Weak signals can stem from multiple points in your protocol [49]:
- Sample Issues: Use freshly isolated cells whenever possible. If using frozen cells, verify that the freezing/thawing process does not damage your target antigen. Confirm that your target protein is expressed at sufficient levels [49].
- Antibody Issues: The antibody may be too dilute. Titrate your antibody to find the optimal concentration and consider increasing the incubation time. For low-expression targets, use a brighter fluorescent dye or a two-step staining method to amplify the signal [49].
- Accessibility Issues: For intracellular targets, ensure your fixation and permeabilization methods are appropriate and do not render the target antigen inaccessible [49].
- Protocol Timing: Perform all steps at 4°C using cold reagents to prevent internalization and degradation [49].
Q3: During the development of a new functional assay, how can we ensure the quality of our membrane protein preparation?
A: A rigorous quality control (QC) system is fundamental. Before beginning functional assays, characterize your protein using the following biophysical methods [50]:
Table: Essential Quality Control Checks for Membrane Protein Preparations
| Attribute to Check | Recommended Biophysical Methods | Acceptance Criteria Examples |
|---|---|---|
| Identity & Purity | LC-MS, SDS-PAGE, Analytical Gel Filtration, Dynamic Light Scattering (DLS) | Single band on gel; monodisperse solution; correct molecular mass [50]. |
| Concentration | Ultraviolet (UV) Spectrum, Bradford Assay | Concentration confirmed via multiple methods (e.g., A280 and Bradford) [50]. |
| Functionality | Functional Activity Assay, ITC, SPR | Binding affinity (Kd) within 2-fold of reference; expected catalytic activity (kcat, Km); stoichiometry (n) ±15% of expected [50]. |
| Stability | Differential Scanning Fluorimetry (DSF), Differential Scanning Calorimetry (DSC) | Defined melting temperature (Tm); good pre- and post-transition baselines [50]. |
Applying a comprehensive QC system like the MSCohort framework, which uses dozens of metrics for individual experiment and cohort-level evaluation, can ensure reproducibility and robustness in your data generation pipeline [51].
Q4: How does the choice of membrane filtration during enzymatic hydrolysis impact the resulting biological activity?
A: The method used to isolate or concentrate the protein fraction post-hydrolysis directly influences functional outcomes. For example, in the enzymatic hydrolysis of potato juice proteins [52]:
- Using an ultrafiltration module (1 kDa cut-off) concentrated proteins and retained valuable minerals, resulting in a hydrolysate (PJPH) with high antioxidant activity.
- A subsequent nanofiltration module (300-500 Da cut-off) further concentrated the protein (cPJPH) and altered the mineral profile.
- The final hydrolysates showed increased antioxidant activity and specific cytotoxicity against cancer cells compared to the starting material. This demonstrates that membrane filtration-assisted hydrolysis can be tailored to enhance specific biological activities of the product [52].
The Scientist's Toolkit: Research Reagent Solutions
Table: Key Reagents for Functional Assays [49]
| Reagent / Solution | Function in the Assay |
|---|---|
| Staining Buffer | Provides an optimal ionic and pH environment for maintaining cell viability and facilitating antibody binding. |
| Blocking Buffer | Contains inert proteins (e.g., BSA) or sera to occupy non-specific binding sites on cells, reducing background signal. |
| Fixative | Preserves the cellular architecture and cross-links proteins at a specific time point, halting biological processes. |
| Permeabilizer | Dissolves cellular membranes, allowing antibodies and other detection reagents to access intracellular targets. |
| Primary Antibodies | Specifically bind to the target protein or antigen of interest with high affinity. |
| Secondary Antibodies | Conjugated to a fluorophore or enzyme, they bind to the primary antibody to enable detection and signal amplification. |
Experimental Workflow and Data Analysis
Below is a generalized workflow for developing and troubleshooting a functional assay, from initial reagent QC to data interpretation.
For transport assays, understanding the specificity of your transporter is key. The following table summarizes the peptide sequence specificity for different TAP (Transporter Associated with Antigen Processing) transporters, illustrating how substrate preferences must be characterized [53].
Table: Peptide Sequence Specificity of TAP Transporters (Based on Direct Transport Assays)
| TAP Transporter | C-terminal Residue Preference | Key Positional Tolerances |
|---|---|---|
| Human TAP1/TAP2 | Permissive; any residue except proline [53]. | Proline not tolerated at positions 1-3. Acidic residue at position 6 or 7 can enhance transport [53]. |
| Rat TAP1/TAP2–A (cima) | Permissive; any residue except proline [53]. | Proline not tolerated at positions 1-3 [53]. |
| Rat TAP1/TAP2–B (cimb) | Restricted; hydrophobic/aromatic residues preferred [53]. | Proline not tolerated at positions 1-3. Does not tolerate G or basic residues at position 2 [53]. |
| Mouse TAP1a/TAP2c | Restricted; hydrophobic/aromatic residues preferred [53]. | Proline not tolerated at positions 1-3 [53]. |
Solving Common Problems: A Practical Guide to Optimizing Stability and Yield
Core Concepts: Why Membrane Proteins Aggregate
1.1 What are the fundamental reasons my solubilized membrane protein aggregates? Membrane protein aggregation often occurs due to a mismatch between the protein's native lipid bilayer environment and the artificial detergent micelle it is placed in during extraction. In their native state, membrane proteins are stabilized by hydrophobic interactions with the lipid bilayer [54]. During detergent extraction, this stabilizing environment is replaced, which can lead to partial unfolding and expose hydrophobic regions that drive non-specific aggregation [55] [56]. Even properly folded proteins can aggregate through detergent-mediated mechanisms, where detergent molecules form bridges between the hydrophilic apical surfaces of multiple protein molecules, creating stable complexes that can persist for extended periods [57].
1.2 How does delipidation during purification contribute to instability? The process of delipidation—the removal of essential native lipids during solubilization and purification—severely impacts protein stability and function [56]. These native lipids often play crucial structural roles, and their loss can destabilize the protein's fold. Eukaryotic membrane proteins appear to be particularly sensitive to lipid removal compared to prokaryotic ones [56]. This is why supplementation with specific lipids during purification often improves stability and reduces aggregation.
Diagnostic Methods: Identifying Aggregation Causes
2.1 What rapid methods can I use to assess aggregation state? Fluorescent Size Exclusion Chromatography (FSEC) provides a powerful, high-throughput method for evaluating membrane protein quality and aggregation state with minimal sample consumption [58]. By tagging your protein with a fluorescent marker (such as GFP), you can monitor its elution profile to identify monodisperse protein versus aggregates. This technique is particularly valuable for comparing different detergent conditions, constructs, and the effects of ligand addition on aggregation state [58].
2.2 How can I quantitatively measure protein stability across different detergents? Differential Scanning Fluorimetry (nanoDSF) with simultaneous static light scattering detection allows you to measure both unfolding temperature (Tm) and aggregation onset (Tagg) in a high-throughput format [56]. This method monitors the intrinsic fluorescence of tryptophan residues during a thermal ramp while simultaneously detecting aggregation via light scattering. By screening across multiple detergents, you can identify conditions that maximize stability and minimize aggregation.
Table 1: Diagnostic Methods for Aggregation Assessment
| Method | What It Measures | Key Output Parameters | Sample Throughput |
|---|---|---|---|
| Fluorescent SEC (FSEC) | Hydrodynamic radius & monodispersity | Elution profile, aggregation peak | Medium (requires purification) |
| nanoDSF | Thermal unfolding & aggregation | Tm (melting temperature), Tagg (aggregation temperature) | High (96-well format) |
| Light Scattering | Particle size & aggregation state | Polydispersity index, aggregation onset | Medium to High |
| CPM Dye Assay | Cysteine exposure upon unfolding | Tm, cooperative unfolding transition | Medium (requires cysteine residues) |
Troubleshooting Guide: Common Aggregation Scenarios
3.1 My protein aggregates immediately after solubilization. What should I do? This typically indicates a poor match between your initial extraction detergent and the protein's stability requirements. Consider these steps:
- Screen alternative detergents: Move beyond traditional DDM to explore newer detergents like Lauryl Maltose Neopentyl Glycol (LMNG) or Glycodiosgenin (GDN), which often provide enhanced stability due to their more rigid structures and improved packing around transmembrane helices [59].
- Include lipid supplements: Add essential lipids during solubilization. Eukaryotic proteins are particularly sensitive to lipid environment [56].
- Test milder conditions: Reduce mechanical stress during extraction and maintain physiological temperatures when possible.
3.2 How can I prevent aggregation during concentration steps? Concentration frequently co-concentrates detergent micelles alongside your protein, leading to instability [60]. Implement these strategies:
- Monitor detergent concentration: Use thin-layer chromatography with iodine vapor staining to quantify detergent levels before and after concentration [60].
- Employ gradual concentration: Step-wise concentration with stability assessment at each stage helps identify the point at which aggregation begins.
- Consider detergent exchange: If concentration is unavoidable, switch to a detergent with smaller micelle size (like OG) before concentration, then exchange back to your preferred detergent.
3.3 What if my protein appears stable initially but aggregates over time? Time-dependent aggregation suggests gradual unfolding or detergent-mediated aggregation. Address this by:
- Conducting long-term stability studies: Use FSEC to monitor samples over days/weeks under different storage conditions [58].
- Testing ligand stabilization: Add substrates, inhibitors, or allosteric modifiers that can stabilize the native conformation [58].
- Evaluating alternative membrane mimetics: Consider transferring to nanodiscs, amphipols, or SMALPs for long-term storage [59].
Detergent Selection & Optimization Strategies
4.1 Which detergents typically provide the best stabilization? While optimal detergent choice is protein-specific, systematic screening has revealed general patterns. Maltoside-based detergents (DDM, DM) often provide good initial stabilization, while newer agents like LMNG frequently show superior performance for many targets [59] [56]. Notably, fos-choline and PEG family detergents tend to be destabilizing for many membrane proteins [56].
Table 2: Detergent Performance for Membrane Protein Stabilization
| Detergent Class | Examples | Stabilization Profile | Considerations |
|---|---|---|---|
| Maltosides | DDM, DM | Good initial stabilization, mild | Large micelle size may hinder crystallization |
| MNG Derivatives | LMNG | Superior stabilization for many targets | Low CMC makes removal difficult |
| Glycosides | GDN, Digitonin | Excellent for Cryo-EM studies | Higher cost, complex structure |
| Neopentyl Glycols | P-GNG | Promising new class, similar to LMNG | Limited usage history |
| Fos-Cholines | Fos-Choline-12 | Often destabilizing | Useful for initial extraction only |
4.2 How do I systematically screen detergent conditions? Establish a high-throughput screening pipeline:
- Start with a broad initial screen: Test 20-30 detergents from different classes using nanoDSF to identify top candidates [56].
- Evaluate monodispersity: Use FSEC to verify that promising detergents from the thermal shift assay also yield monodisperse protein [58].
- Assess functionality: Ensure that stabilizing conditions maintain biological activity through functional assays.
- Optimize lipid supplements: Identify essential lipids that further enhance stability in your chosen detergent [56].
Lipid Supplementation Approaches
5.1 Which lipids should I add to stabilize my membrane protein? The specific lipid requirements vary by protein class, but some general guidelines emerge:
- Transporters & GPCRs: Often require cholesterol or cholesterol analogs for optimal stability and function [56].
- Respiratory complexes: Typically need cardiolipin or other anionic phospholipids [59].
- General stabilization: Phosphatidylcholine, phosphatidylglycerol, or phosphatidylethanolamine mixtures can benefit many proteins.
5.2 How should I deliver lipid supplements to my protein? Lipid supplementation strategies include:
- Pre-formed lipid mixtures: Add during purification to maintain a lipid-like environment [56].
- Specific lipid reconstitution: Identify essential lipids through mass spectrometry of purified native complexes, then supplement with these specific lipids [59].
- Native nanodisc approaches: Use SMA or DIBMA polymers to create "native nanodiscs" that preserve the original lipid environment [59].
Experimental Protocols
6.1 High-Throughput Detergent Screening Protocol
This protocol adapts the methodology from [56] for systematic detergent evaluation:
- Prepare protein sample: Solubilize and purify your membrane protein in a standard detergent (typically 1-2% DDM).
- Set up detergent screen: Create a 96-well plate with 0.05-0.1% solutions of test detergents in appropriate buffer.
- Dilute protein: Add small aliquots of your protein sample to each detergent condition (final protein concentration 0.1-0.5 mg/mL).
- Run nanoDSF measurement: Use a nanoDSF instrument to monitor intrinsic tryptophan fluorescence (350 nm/330 nm ratio) while ramping temperature from 20°C to 95°C at 1°C/minute.
- Analyze results: Determine Tm from the inflection point of the unfolding curve and Tagg from the light scattering signal.
- Validate with FSEC: For promising conditions, analyze by FSEC to confirm monodispersity [58].
6.2 Detergent Removal and Exchange Protocol
When excess detergent causes aggregation in downstream applications [60]:
Choose removal method based on detergent properties:
- For high-CMC detergents (>5 mM): Use dialysis against detergent-free buffer with multiple buffer changes.
- For low-CMC detergents: Employ polystyrene beads (Bio-Beads) with optimization of bead-to-sample ratio.
- For rapid removal: Use detergent-removal spin columns following manufacturer's instructions.
Monitor detergent concentration using TLC with iodine vapor staining [60]:
- Spot samples alongside detergent standards of known concentration.
- Develop in a sealed chamber with iodine crystals.
- Quantify by laser densitometry or ImageJ analysis.
- Calculate concentration from a standard curve.
Verify protein stability after detergent modification using FSEC and activity assays.
The Scientist's Toolkit
Table 3: Essential Reagents for Aggregation Prevention
| Reagent/Category | Specific Examples | Function & Application |
|---|---|---|
| Stabilizing Detergents | LMNG, GDN, DDM | Maintain native fold during & after extraction |
| Screening Platforms | nanoDSF, FSEC | Identify optimal stabilizing conditions |
| Detergent Removal | Bio-Beads, HiPPR Columns | Reduce excess detergent for downstream applications |
| Lipid Supplements | CHS, Cardiolipin, E. coli Polar Lipids | Restore essential native lipid interactions |
| Membrane Mimetics | Nanodiscs (MSP), SMALPs, Amphipols | Provide lipid-bilayer-like environment for long-term stability |
Aggregation Pathways & Quality Assessment Workflow
Frequently Asked Questions
Q1: How much detergent should I use for initial solubilization? For initial extraction, use detergent concentrations at 100x CMC to ensure complete membrane solubilization [60]. During purification, gradually reduce concentration while monitoring stability. For final samples, aim for the minimal detergent concentration that maintains solubility, typically with detergent:protein ratios around 10:1 (w/w) for complete delipidation [54].
Q2: My protein only stays soluble in harsh denaturing detergents. Can I recover native structure? Yes, through careful refolding protocols. precipitate the protein to remove denaturing detergent, then refold in a buffer containing milder stabilizing detergents and essential lipid supplements [60]. However, this requires extensive optimization and functional validation at each step.
Q3: How can I distinguish between protein aggregation and detergent-mediated aggregation? Detergent-mediated aggregation often shows concentration dependence and occurs even with properly folded proteins [57]. True protein aggregation typically correlates with unfolding transitions in thermal shift assays. FSEC can help distinguish these—detergent-mediated aggregates may dissociate under different detergent conditions, while protein aggregates persist.
Q4: Are newer detergent alternatives like SMALPs and nanodiscs better for preventing aggregation? These membrane mimetics often provide superior stability by preserving a lipid-bilayer-like environment [59]. SMALPs spontaneously encapsulate membrane proteins with surrounding native lipids, while nanodiscs provide a controlled lipid environment. However, both require initial detergent extraction and additional optimization, making them more suitable for downstream applications rather than initial solubilization [59].
Membrane proteins represent crucial therapeutic targets, but studying them requires extraction from their native lipid environment. Detergents are amphipathic molecules that serve as indispensable tools for this process, solubilizing and stabilizing membrane proteins by forming micelles that mimic the membrane environment [59]. The hydrophobic tails of detergent molecules form a core that integrates with hydrophobic protein regions, while hydrophilic head groups interact with the aqueous solution to maintain solubility [59]. Achieving the optimal balance between detergent concentration, sample homogeneity, and practical cost considerations presents a significant challenge for researchers. This technical support center addresses these critical experimental parameters through targeted troubleshooting guides and detailed FAQs designed specifically for membrane protein researchers working within quality assessment frameworks.
Troubleshooting Guides: Addressing Common Experimental Challenges
Detergent Selection and Optimization
Problem: Poor protein stability or aggregation during purification
| Possible Cause | Diagnostic Tests | Solution |
|---|---|---|
| Inappropriate detergent harshness | Compare activity pre/post solubilization; test multiple detergent classes | Start with "mild" non-ionic detergents (DDM) or advanced formulations (LMNG) [59] |
| Critical Micelle Concentration (CMC) too high | Measure CMC; assess protein stability at concentrations above/below CMC | Switch to low-CMC detergents (LMNG) or add supplemental lipids/cholesterol hemisuccinate |
| Incompatibility with downstream analysis | Test protein function/activity in final detergent | Utilize newer detergent classes (MNG, P-GNG, GDN) with improved stability profiles [59] |
Problem: High experimental costs associated with detergent usage
| Cost Factor | Cost-Saving Strategy | Implementation Considerations |
|---|---|---|
| Premium detergent pricing | Optimize concentration through systematic titration | Balance protein stability against minimal effective concentration |
| Buffer incompatibility requiring re-optimization | Standardize detergent panels across multiple projects | Leverage bulk purchasing for commonly used detergents |
| Failed experiments due to detergent issues | Implement rigorous pre-screening protocols | Utilize small-scale stability tests before large-scale purification |
Analytical Interference and Compatibility Issues
Problem: Detergent interference with protein quantification assays
| Assay Method | Common Interfering Detergents | Solutions and Workarounds |
|---|---|---|
| BCA and Micro BCA Assays | Reducing agents, chelators, strong acids/bases [61] | Dilute samples, dialyze/desalt, or use precipitation methods |
| 660 nm Assay | Ionic detergents [61] | Test detergent tolerance, use alternative compatible detergents |
| Pierce Bradford Assay | Detergents [61] | Dilute samples substantially or switch to BCA method |
| Modified Lowry Assay | Detergents, reducing agents, chelators [61] | Implement precipitation protocols to remove interferents |
| Qubit Protein Assay | Additional detergents beyond kit formulation [61] | Follow specific volume recommendations and calibration procedures |
Problem: Incompatibility with structural biology techniques
Cryo-EM Challenges: Detergents can cause issues with vitreous ice formation, protein orientation, and background noise [59]. Low-CMC detergents like LMNG produce fewer free detergent molecules, improving image quality [59]. Consider alternative environments like nanodiscs, amphipols, or SMALPs for particularly challenging targets [59].
Frequently Asked Questions (FAQs)
Q1: What are the key considerations when selecting a detergent for a new membrane protein target?
Begin with mild non-ionic detergents like DDM, which is widely successful for many membrane proteins [59]. Consider newer MNG-based detergents like LMNG that often provide enhanced stability and smaller, more uniform micelles [59]. Test multiple detergent classes systematically while monitoring protein stability and function.
Q2: How does Critical Micelle Concentration (CMC) affect my experiments?
CMC determines the concentration at which detergent molecules form micelles. Below CMC, your protein may precipitate; above CMC, excess detergent molecules are present. Low-CMC detergents (like LMNG) provide stability with fewer free detergent molecules, which is particularly beneficial for techniques like Cryo-EM [59].
Q3: What strategies can help reduce detergent-related costs without compromising quality?
Implement small-scale screening (50-100μL scale) to identify optimal detergents and concentrations before scaling up. Consider detergent recycling during purification when appropriate. Purchase in bulk for frequently used detergents, and explore generic alternatives to proprietary formulations where possible.
Q4: How can I troubleshoot detergent interference with protein quantification assays?
First, identify which assay components are incompatible with your detergent [61]. Simple dilution may reduce interference while maintaining detectable protein levels. Alternatively, implement protein precipitation protocols using acetone or TCA to remove interfering substances before resuspending the pellet in compatible buffer [61].
Q5: When should I consider alternatives to traditional detergents?
Consider nanodiscs, amphipols, or SMALPs when: (1) your protein is unstable in all detergents tested; (2) you need to preserve native lipid interactions; or (3) you require a more membrane-like environment for functional studies [59]. Note that these methods often still require initial detergent solubilization before reconstitution.
Experimental Protocols: Key Methodologies
Systematic Detergent Screening Protocol
- Create a diversified detergent panel: Include traditional (DDM, OG), advanced (LMNG, GDN), and ionic detergents at 1-2× CMC concentrations.
- Small-scale solubilization: Test each detergent at 1-2 mg detergent/mg protein in 50-100μL scale for 1-2 hours.
- Centrifugation step: Remove insoluble material (100,000×g, 30 minutes).
- Analyze supernatant: Assess protein content (e.g., FSEC), monodispersity (DLS), and stability (activity assays over 24-48 hours).
- Scale up promising candidates: Advance 2-3 top performers to larger-scale purification.
Detergent Tolerance Testing for Protein Assays
- Prepare standard curve: Using BSA or target protein in compatible buffer.
- Spike-in experiment: Add your detergent to both standards and blanks at the concentration present in your samples.
- Compare curves: Standard curves with and without detergent will reveal interference.
- Implement correction: If interference is minimal and consistent, use spiked standards for quantification.
- Alternative approach: If interference is substantial, implement protein precipitation before assay.
Research Reagent Solutions: Essential Materials
| Reagent Category | Specific Examples | Function and Application Notes |
|---|---|---|
| Traditional Detergents | DDM, β-OG [59] | Mild, non-ionic detergents; good starting points for novel targets |
| Advanced Maltose-Based | LMNG, MNG derivatives [59] | Enhanced stability, smaller micelles, low CMC; superior for structural studies |
| Specialized Detergents | GDN, Digitoxin [59] | Often useful for challenging targets like GPCRs and transporters |
| Alternative Environments | Nanodiscs (MSP), Amphipols, SMALPs [59] | Provide more native-like membrane environment; useful for unstable targets |
| Quantification Kits | BCA, Bradford, Qubit Assays [61] | Varying detergent compatibility; selection depends on detergent used |
Workflow Visualization
Figure 1: Membrane Protein Preparation Workflow. This diagram outlines the iterative process for optimizing detergent conditions to achieve high-quality membrane protein preparations.
In membrane protein topology studies, the integrity of the plasma membrane is a critical experimental parameter. Compromised membranes can lead to mislocalization of epitopes, false-positive staining, and ultimately, incorrect topological models. Propidium iodide (PI) serves as a essential tool for monitoring membrane integrity, providing a simple and reliable method to distinguish between intact and compromised cells during immunofluorescence and flow cytometry experiments. This guide provides detailed protocols and troubleshooting advice to ensure accurate assessment of membrane integrity within the broader context of quality assessment for membrane protein preparations research.
Experimental Protocols
Basic Propidium Iodide Staining Protocol for Flow Cytometry
This protocol outlines the steps for using PI to assess cell viability and membrane integrity in cell suspensions [62].
- Reagents Required: Phosphate-Buffered Saline (PBS), Flow Cytometry Staining Buffer, PI Staining Solution (10 µg/mL in PBS) [62].
- Materials Required: FACS tubes (5 mL round-bottom polystyrene tubes), pipettes and tips, centrifuge, vortex [62].
- Cell Harvesting and Washing: Harvest cells and aliquot up to 1 x 10⁶ cells per 100 µL into FACS tubes. Wash cells by adding 2 mL of PBS, centrifuging at 300 x g for 5 minutes, and decanting the supernatant from the cell pellet. Repeat this wash step a second time [62].
- Resuspension: Resuspend the washed cell pellet in 100 µL of Flow Cytometry Staining Buffer [62].
- Staining and Analysis: Add 5-10 µL of PI staining solution to the cell suspension just before analysis on the flow cytometer. Mix gently and incubate for approximately 1 minute in the dark. Do not wash the cells after adding PI. Analyze immediately, setting the stop count on the viable cell population [62].
PI Staining for Fixed or Permeabilized Cells in Cell Cycle Analysis
This protocol is used when PI is employed for DNA content analysis, which requires the dye to access the nucleus. It highlights the importance of fixation and RNase treatment [63].
- Reagents Required: PBS, 70% Ethanol (prepared with distilled water, not PBS), Propidium Iodide stock solution (50 µg/mL), Ribonuclease I (RNase) stock solution (100 µg/mL) [63].
- Materials Required: Centrifuge, vortex.
- Cell Harvesting and Fixation: Harvest cells and wash in PBS. Gently centrifuge and remove supernatant. Fix the cell pellet by adding cold 70% ethanol drop-wise while gently vortexing the tube. Fix for 30 minutes at 4°C [63].
- Washing: Wash the fixed cells twice in PBS by centrifuging at approximately 850 x g. Take care when discarding the supernatant to avoid losing the pellet [63].
- RNase and PI Treatment: Add 50 µL of the 100 µg/mL RNase stock to the cell pellet to digest RNA. Add 200 µL of the 50 µg/mL PI stock solution [63].
- Analysis: Analyze the cells by flow cytometry. Use forward scatter vs. side scatter to identify cells, and pulse processing (pulse width vs. pulse area) to exclude cell doublets and clumps. The PI signal, measured with a ~605 nm bandpass filter, will reflect DNA content [63].
Confocal Microscopy for Validating Membrane Integrity
Confocal microscopy provides a powerful visual method to confirm flow cytometry findings and investigate anomalies, such as uneven staining or false positives [64].
- Principle: Confocal microscopy uses a pinhole aperture to block out-of-focus light, generating high-resolution optical sections of a sample. This allows for precise localization of PI staining within a cell [65].
- Sample Preparation: Cells are typically labeled with fluorescent dyes (like PI) via direct or indirect staining methods. For topology studies, plasma membrane integrity is monitored by co-incubating live, unpermeabilized cells with PI. Immunostaining analysis should be restricted to the PI-negative cell population to ensure the plasma membrane is intact [14].
- Key Considerations: Use minimal laser power to reduce photobleaching and phototoxicity. Ensure proper calibration and alignment of the system. Select fluorophores with minimal excitation/emission spectra overlap to prevent bleed-through [65].
Troubleshooting Guides
Frequently Encountered Problems and Solutions
| Problem | Possible Causes | Recommended Solutions |
|---|---|---|
| High Background/Non-specific PI Staining | PI binding to RNA, Dead/dying cells in sample, Antibody non-specificity [64] [66] | Treat samples with RNase to degrade RNA [63]; Ensure healthy cell culture and quick processing; Include proper controls (unstained, single stains) [66]. |
| Loss of Signal/Weak PI Fluorescence | Low laser power, Misaligned optics, Incorrect detector settings [67] | Check and align laser and optics; Ensure PMT voltage/gain is set appropriately for PI detection [67]. |
| High Percentage of PI-Positive Cells | Overly harsh cell harvesting, Toxic culture conditions, Ethanol fixation (in DNA content protocols) [63] | Use gentle detachment methods (e.g., optimized trypsinization); Check culture health and conditions; Understand that fixation will permeabilize all cells [63]. |
| Unexpected Spectral Overlap (Compensation Issues) | Broad emission spectrum of fluorophores, Incorrect compensation settings [66] [67] | Use fluorophores with minimal spectral overlap; Run single-stain controls and adjust compensation properly on the cytometer [66]. |
| Variability in Results Between Experiments | Inconsistent cell preparation, PI solution degradation, Changes in instrument settings [67] | Standardize cell harvesting and staining protocols; Protect PI from light, aliquot, and store properly; Perform regular instrument calibration and quality control [67]. |
Addressing False Positives in PI Staining
A significant challenge in PI staining is the occurrence of false positive events, which can exceed 40% in some conventional protocols [64].
- Cause: A primary cause is the binding of PI to RNA within the cytoplasmic compartment. This is particularly common in larger cells with a smaller nuclear-to-cytoplasmic ratio [64].
- Solution: Incorporation of an RNase treatment step is crucial to eliminate RNA-associated fluorescence, ensuring that the signal originates solely from DNA [63].
- Validation: For complex samples like bacterial biofilms, where extracellular nucleic acids can cause a false dead layer of staining, viability results should be validated by an alternative method, such as confocal laser scanning microscopy (CLSM) [64].
Frequently Asked Questions (FAQs)
Q1: Why is membrane integrity so critical in membrane protein topology studies? Membrane integrity is fundamental because it preserves the native asymmetry of the plasma membrane. In topology studies, antibodies are used to determine whether an epitope is extracellular or intracellular. If the membrane is compromised, antibodies can access intracellular epitopes even in live cell conditions, leading to a completely misinterpreted topology model [14].
Q2: My PI-stained sample shows a high level of background fluorescence. What is the most likely cause? The most common cause of high background is the binding of PI to intracellular RNA, not just DNA. To resolve this, incorporate an RNase treatment step into your staining protocol to digest RNA and significantly improve signal specificity [63].
Q3: Can I use PI for live-cell topology studies? PI is used in live-cell topology studies specifically as an exclusion dye. Live, intact cells with an impermeable membrane will exclude PI and be negative. Only cells with compromised membranes (dead or dying) will be PI-positive. Therefore, for topology analysis, you should only analyze the live, PI-negative cell population to ensure your results are based on intact membranes [14].
Q4: How does the use of PI differ between viability assessment and cell cycle analysis? For simple viability assessment, cells are not fixed or permeabilized, and PI is added just before analysis to identify dead cells with leaky membranes. For cell cycle analysis, all cells are first fixed and permeabilized (e.g., with ethanol) to allow PI uniform access to the nucleus. An RNase step is also critical here to ensure the signal is DNA-specific [62] [63].
Q5: What are the safety considerations when working with propidium iodide? Propidium iodide is a suspected carcinogen and may cause skin, eye, and respiratory irritation. It should be handled with care, using appropriate personal protective equipment (PPE). The dye must be disposed of in accordance with local hazardous waste regulations [62] [64].
The Scientist's Toolkit: Research Reagent Solutions
| Item | Function in Experiment | Key Considerations |
|---|---|---|
| Propidium Iodide (PI) | Membrane-impermeant DNA dye used to identify dead/dying cells with compromised membranes [62] [64]. | Suspected carcinogen; requires safe handling; also binds RNA, necessitating RNase treatment for DNA-specific staining [64] [63]. |
| Ribonuclease (RNase) | Enzyme that degrades RNA to prevent non-specific PI staining and reduce background fluorescence [63]. | Essential for accurate DNA content analysis and reducing false positives in viability assays [64] [63]. |
| Flow Cytometry Staining Buffer | Buffer used to resuspend and stain cells; often contains protein (e.g., BSA) to reduce non-specific antibody binding [62]. | Helps maintain cell health and reduce background during staining procedures. |
| Fixatives (e.g., Paraformaldehyde, Ethanol) | Used to permeabilize cells for intracellular staining, such as DNA content analysis with PI [63]. | Aldehydes (PFA) preserve structure but may require subsequent permeabilization; alcohol (ethanol) both fixes and permeabilizes but can damage some epitopes [63]. |
| Antibodies (Primary & Fluorescently-Labeled Secondary) | Used to bind specific epitopes on the membrane protein of interest for topology mapping [14]. | In intact cell staining, binding indicates an extracellular epitope; binding only in permeabilized cells indicates an intracellular epitope [14]. |
Experimental Workflow for Membrane Integrity Assessment
The following diagram illustrates the key decision points and steps in a typical experiment using PI to assess membrane integrity for topology studies.
Figure 1. Logical workflow for using Propidium Iodide (PI) to gate intact cells for membrane protein topology studies. PI-positive cells with compromised membranes are excluded from analysis to ensure results reflect native protein orientation.
Troubleshooting Guides and FAQs
Cell Health and Viability
Q: My mammalian cells are not growing well after passaging, which is affecting my protein yield. What could be the cause?
A: Poor cell growth post-passaging can stem from several issues. First, check that you are using the correct growth medium and that your fetal bovine serum (FBS) is of high quality and not from a degraded lot [68]. Ensure you are passaging cells when they are in the log-phase of growth, before they reach confluency [68]. Also, confirm that you are using a low-passage number of cells, as cells passaged too many times can lose their growth potential [68]. Finally, always use pre-warmed growth medium as recommended by the supplier for thawing and feeding cells [68].
Q: I suspect my cell culture is contaminated with mycoplasma. How can I address this?
A: Mycoplasma contamination is subtle but can severely impact protein quality and cell health. Segregate the suspect culture immediately to prevent spread [68]. Decontamination is very difficult; often the culture must be discarded. For irreplaceable cultures, antibiotics like Ciprofloxacin or Plasmocin can be attempted, but the treated culture must be quarantined and rigorously tested until confirmed clean [68]. Implement regular testing protocols to catch future contaminations early.
Extraction and Lysis
Q: My protein extraction efficiency is low, especially for membrane proteins. How can I improve cell lysis?
A: For comprehensive proteomic analysis, including membrane proteins, a combination of thermal and mechanical disruption has been shown to be highly effective. One optimized protocol is to use an SDS-based lysis buffer (e.g., containing 4% SDS) coupled with both boiling and ultrasonication [69]. Resuspend the cell pellet in SDT lysis buffer, incubate in a 98°C water bath for 10 minutes, cool, and then sonicate on ice (e.g., 5 seconds on, 8 seconds off per cycle for a total of 5 minutes at 70% amplitude) [69]. This method, known as SDT-B-U/S, enhances protein recovery and is particularly effective for membrane proteins like OmpC [69].
Q: My extracted proteins are aggregating or clumping. What should I do?
A: Cell aggregation can often be prevented by ensuring thorough dissociation into a single-cell suspension during passaging [70]. For the extraction itself, ensure thorough cell dissociation during the lysis step. The use of detergents like SDS in the lysis buffer, combined with a reducing agent like DTT, helps to denature proteins and prevent aggregation [69]. Also, avoid generating excessive heat during sonication by always performing it on ice.
Purification and Downstream Processing
Q: I am purifying a recombinant protein from a complex feedstock with many host cell proteins. What strategies can I use?
A: Purifying recombinant proteins from complex feedstocks, such as those resulting from cell disruption (as opposed to secretion), is challenging due to abundant host cell proteins (HCPs), DNA, and other impurities [71]. Scalable and cost-efficient purification requires strategies that can handle this complexity. Exploring different chromatography resins and sequences is key. Furthermore, integrating Host Cell Protein (HCP) profiling into your process design can be highly informative. By characterizing the specific HCPs present, you can better select purification steps that effectively remove these critical impurities, thereby enhancing final product purity [71].
Q: The pH of my culture medium shifts rapidly, could this affect my recombinant protein?
A: Yes, rapid pH shifts can negatively impact protein stability and quality, potentially leading to issues like fragmentation, aggregation, or charge variants [72]. To correct a rapid pH shift, ensure the CO₂ tension in your incubator matches the sodium bicarbonate concentration in your medium [68]. For a medium with 2.0 to 3.7 g/L sodium bicarbonate, use 5–10% CO₂, respectively [68]. You can also add HEPES buffer to a final concentration of 10–25 mM for additional buffering capacity, and ensure tissue culture flask caps are loosened one-quarter turn to allow for gas exchange [68].
Experimental Protocols and Data
Detailed Methodology: Plasma Membrane Protein Enrichment
This protocol is designed for the enrichment of plasma membrane proteins from mammalian cells (including cell lines, CDX/PDX, and primary tissues) for downstream mass spectrometry analysis [73].
- Cell Lysis: Lyse cells using a hypotonic buffer and a Dounce homogenizer to mechanically disrupt the cell membrane while keeping organelles intact. Protease and phosphatase inhibitors should be added to the lysis buffer to preserve protein integrity.
- Density Gradient Ultracentrifugation: The cell lysate is then layered onto a discontinuous sucrose density gradient (e.g., ranging from 10% to 50% sucrose). The gradient is subjected to high-speed ultracentrifugation for several hours.
- Membrane Isolation: Following centrifugation, the plasma membrane fraction will migrate to a specific density interface within the gradient. This band is carefully collected by pipetting.
- Preparation for Downstream Analysis: The enriched plasma membrane fraction is washed to remove sucrose and can be solubilized for direct proteomic analysis or further purified [73].
Detailed Methodology: Enhanced Protein Extraction via SDT-B-U/S
This protocol, adapted for mammalian cells, is based on the highly effective SDT-B-U/S method evaluated in bacterial systems [69].
- Harvesting and Washing: Harvest mammalian cells by centrifugation. Wash the cell pellet three times with cold phosphate-buffered saline (PBS) to remove residual culture medium.
- Boiling Lysis: Thoroughly resuspend the cell pellet in SDT lysis buffer (4% w/v SDS, 100 mM DTT, 100 mM Tris-HCl, pH 7.6). Vortex the suspension and incubate it in a 98°C water bath for 10 minutes to ensure complete cell lysis and protein denaturation.
- Ultrasonication: After cooling the lysate on ice, subject it to ultrasonication on ice using an ultrasonic probe. A typical setting is 70% amplitude for a total of 5 minutes, using a cycle of 5 seconds on and 8 seconds off to prevent overheating.
- Clarification: Centrifuge the lysate at 10,000 × g for 10 minutes at 4°C to remove insoluble cell debris. Collect the supernatant, which contains the solubilized proteins.
- Protein Precipitation (Optional): For buffer exchange or concentration, proteins can be precipitated by adding four volumes of pre-cooled acetone and incubating overnight at -20°C. Pellet proteins by centrifugation, wash with cold acetone, and resuspend in an appropriate buffer for downstream applications [69].
Table 1: Impact of Extraction Methods on Protein Identification
| Extraction Method | Description | Unique Peptides Identified (E. coli, DDA) | Unique Peptides Identified (S. aureus, DDA) | Technical Replicate Correlation (DIA, R²) |
|---|---|---|---|---|
| SDT-B-U/S [69] | Boiling + Ultrasonication | 16,560 | 10,575 | 0.92 |
| SDT-U/S [69] | Ultrasonication only | Data suggests lower than SDT-B-U/S | Data suggests lower than SDT-B-U/S | Lower than SDT-B-U/S |
| SDT-B [69] | Boiling only | Data suggests lower than SDT-B-U/S | Data suggests lower than SDT-B-U/S | Lower than SDT-B-U/S |
| SDT-LNG-U/S [69] | Liquid Nitrogen Grinding + U/S | Data suggests lower than SDT-B-U/S | Data suggests lower than SDT-B-U/S | Lower than SDT-B-U/S |
Table 2: Effect of Genetic and Vector Optimizations on Recombinant Protein Expression in CHO Cells
| Optimization Strategy | Specific Change | Effect on Recombinant Protein Expression |
|---|---|---|
| Regulatory Elements [74] | Addition of Kozak sequence upstream of gene | Increased eGFP expression 1.26-fold vs. control |
| Regulatory Elements [74] | Addition of Kozak + Leader sequence upstream of gene | Increased eGFP expression 2.2-fold vs. control |
| Regulatory Elements [74] | Addition of Kozak sequence for SEAP protein | Increased stable SEAP expression 1.49-fold vs. control |
| Apoptosis Inhibition [74] | Knockout of Apaf1 gene using CRISPR/Cas9 | Increased recombinant protein production (specific fold not stated) |
Workflow and Pathway Visualizations
Mammalian Cell Protein Extraction Workflow
Apoptosis Inhibition to Boost Protein Yield
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents for Mammalian Cell Protein Extraction and Purification
| Reagent / Material | Function / Explanation |
|---|---|
| SDT Lysis Buffer [69] | A buffer containing SDS (a potent ionic detergent) and DTT (a reducing agent). It efficiently disrupts lipid membranes and denatures proteins, facilitating the solubilization of hydrophobic membrane proteins. |
| Sucrose Gradient [73] | Used in density gradient ultracentrifugation to separate cellular components, like the plasma membrane, based on their buoyant density for enrichment prior to extraction. |
| Ultrafiltration Membranes [75] | Membranes with specific molecular weight cut-offs (e.g., 5-50 kDa) are used to concentrate protein samples and exchange buffers, removing salts and small molecules. |
| CRISPR/Cas9 System [74] | A gene-editing tool used for cell line engineering, such as knocking out pro-apoptotic genes (e.g., Apaf1) to enhance cell viability and recombinant protein yield during production. |
| Kozak & Leader Sequences [74] | Regulatory elements added to expression vectors upstream of the target gene. They enhance the translation initiation efficiency of mRNA, leading to higher levels of recombinant protein production. |
Beyond the Basics: Advanced Validation and Cross-Methodological Corroboration
FAQs on Core Principles and Applications
Q1: What are the key strengths of X-ray crystallography and cryo-EM for membrane protein structure determination?
X-ray crystallography is particularly well-suited for obtaining precise atomic coordinates of membrane proteins under a few hundred kDa in size and is better equipped to provide high-resolution dynamic information as a function of time, temperature, and pressure. In contrast, cryo-EM excels at determining the structures of larger, potentially more disordered macromolecular assemblies and avoids the crystallization bottleneck entirely, which is a significant advantage for many membrane proteins. Furthermore, cryo-EM offers increasing insight into conformational and energy landscapes as algorithms to deconvolute conformational heterogeneity become more advanced [76].
Q2: Why is the membrane mimetic environment critical for validating membrane protein structures?
The structure of membrane proteins is known to be sensitive to the membrane mimetic environment. Functional assays performed in lipid bilayers validate the protein construct but do not validate the structure determined in a detergent environment. Detergent micelles can induce structural perturbations, such as outward curvature of transmembrane helices, lengthened hydrogen bonds, and poor helix packing with large cavities, which may not represent the native state in a lipid bilayer. Therefore, validation techniques like oriented sample solid-state NMR (OS ssNMR), performed in lipid bilayers, are essential for assessing whether a structure is native-like [77].
Q3: What is the "gold-standard" for estimating the resolution of a cryo-EM map?
The recommended method is gold-standard Fourier Shell Correlation (FSC). This involves splitting the particle images into two independent sets and reconstructing two separate 3D volumes. The Fourier components of these maps are compared, and the correlation is calculated in spherical shells corresponding to different resolutions. The resolution is widely estimated as the frequency at which the FSC curve drops below a threshold of 0.143. This method helps prevent overestimation of resolution from overfitting [78].
Q4: What are the essential validation criteria for a high-quality X-ray crystallographic structure?
The quality of an X-ray structure is assessed using several key criteria [79]:
- Resolution: Higher resolution (lower numerical value) yields a more accurate atomic model.
- R-factors: The R-factor and R-free indicate how well the model fits the experimental data. Lower values represent a better fit.
- Model Geometry: This includes checks for deviations from ideal bond lengths and bond angles.
- Ramachandran Plot: A good quality structure has a high percentage of residues in the most favored and allowed regions, with few or no outliers.
- B-factors (Temperature Factors): These indicate the vibrational motion or positional disorder of atoms. Well-ordered regions have lower B-factors.
Q5: How can local errors in a protein model be identified?
Local errors can be detected through several methods. In cryo-EM, local resolution estimation can reveal flexible or disordered regions that appear more diffuse. In both X-ray and cryo-EM models, validation tools can identify steric clashes (atoms too close together), side-chain rotamer outliers, and unusual protein backbone torsion angles (Ramachandran plot outliers). For a more global perspective, complex network analysis of the residue contact map can pinpoint locally misfolded regions by identifying residues with an unusually low number of contacts or abnormally long/short paths between residues [80].
Troubleshooting Guides
Table 1: Troubleshooting Common Issues in Cryo-EM
| Issue | Possible Causes | Recommended Solutions |
|---|---|---|
| Low Resolution Map | Particle images are small or have low signal-to-noise ratio; preferred orientation of particles; inaccurate particle alignment [78]. | Use direct electron detectors and movie frames with motion correction; employ advanced particle-picking and denoising algorithms; consider data collection at a tilt angle to mitigate preferred orientation [78]. |
| Over-refinement ("Einstein from noise") | The refined model or map is fitted to noise in the particle images rather than the true signal [78]. | Use gold-standard FSC with two independent reconstructions; perform high-resolution noise substitution or phase randomization to detect overfitting [78]. |
| Resolution Anisotropy | Particles have preferred orientations, leading to features not being resolved equally well in all directions [78]. | Collect data with the specimen tilted; use 3D classification to identify and separate populations of particles with different orientations [78]. |
| Poor Map Quality in Specific Regions | Local flexibility or disorder in the macromolecule; radiation damage to specific residues [78]. | Use 3D variability analysis or classification to isolate conformational states; evaluate local resolution with tools like ResMap or MonoRes [78]. |
Table 2: Troubleshooting Common Issues in X-ray Crystallography
| Issue | Possible Causes | Recommended Solutions |
|---|---|---|
| High R-factor and R-free | Imperfect packing in the crystal lattice (disorder); high solvent content; internal flexibility of the protein; errors in the model [79]. | Improve crystal quality to reduce disorder; ensure proper refinement with restraints/constraints; verify model building in electron density maps, especially for flexible loops and side chains [79]. |
| Poor Electron Density | High flexibility or disorder of the protein region; low resolution of the diffraction data; radiation damage [79]. | If due to flexibility, the model may need to be truncated or represented with multiple conformations; improve crystal quality to achieve higher resolution data; use cryo-cooling to reduce radiation damage [76] [79]. |
| Ramachandran Plot Outliers | Incorrect protein backbone torsion angles; errors in model building, often in loops [79]. | Manually rebuild the problematic residues to fit the electron density and achieve favorable torsion angles; use validation tools to identify and correct outliers during refinement [79]. |
| Weak or No Diffraction | Poor crystal quality; small crystal size; disorder within the crystal [81]. | Optimize crystallization conditions; use micro-focus beamlines for small crystals; consider different crystal forms or constructs (e.g., thermostabilized mutants for membrane proteins) [81] [77]. |
Essential Experimental Protocols
Protocol 1: Gold-Standard Resolution Estimation in Cryo-EM
Objective: To accurately estimate the resolution of a cryo-EM reconstruction while preventing overfitting. Methodology:
- Particle Splitting: After particle picking and extraction, randomly split the entire dataset of particle images into two independent halves [78].
- Independent Reconstruction: Process the two half-sets completely independently from each other to generate two separate 3D reconstructions (Map A and Map B). This independence is crucial [78].
- Fourier Shell Correlation (FSC):
- Resolution Thresholding: The resolution of the map is commonly reported as the value where the FSC curve crosses the 0.143 threshold [78].
- Overfitting Check (Phase Randomization): To test for overfitting, substitute the high-resolution phases in the original particle images with random phases beyond a certain frequency (e.g., 75% of the expected resolution). Recalculate the FSC between the two new maps. A sharp drop in this FSC indicates no overfitting [78].
Protocol 2: Validating a Membrane Protein Structure Using a Lipid Bilayer-Based Technique
Objective: To assess whether a membrane protein structure solved in detergents (by X-ray or cryo-EM) is native-like by comparing it to data acquired in a lipid bilayer environment. Methodology (Using Oriented Sample Solid-State NMR - OS ssNMR):
- Sample Preparation: Reconstitute the purified membrane protein into a liquid crystalline lipid bilayer suitable for OS ssNMR [77].
- Data Collection: Collect OS ssNMR spectra (e.g., ¹⁵N chemical shift/PISA wheel patterns) from the protein in the lipid bilayer [77].
- Prediction from Atomic Coordinates: Using the atomic model from X-ray or cryo-EM, predict the expected OS ssNMR spectrum based on the protein's structure and orientation within the bilayer [77].
- Comparison and Validation: Compare the experimentally observed OS ssNMR spectrum with the spectrum predicted from the atomic coordinates. A close match between the observed and predicted data provides strong evidence that the structure is native-like and has been validated in a membrane environment [77].
Workflow and Relationship Visualizations
Cryo-EM Single Particle Analysis Workflow
Integrated Structural Validation Pathway
The Scientist's Toolkit: Key Research Reagents and Materials
Table 3: Essential Reagents for Membrane Protein Structural Biology
| Reagent / Material | Function in Experiments |
|---|---|
| Lipidic Cubic Phase (LCP) | A membrane mimetic used for crystallizing membrane proteins for X-ray crystallography, providing a more native-like environment than detergent micelles [77]. |
| Detergents (e.g., DPC, DDM) | Amphipathic molecules used to solubilize membrane proteins from the lipid bilayer for purification and structural studies in solution NMR or cryo-EM [77]. |
| Selenomethionine | An amino acid used for experimental phasing in X-ray crystallography (via SAD/MAD) by incorporating selenium atoms, which are strong anomalous scatterers, into the protein [76]. |
| Monoolein | A lipid commonly used to form the lipidic cubic phase matrix for in meso crystallization of membrane proteins [77]. |
| Vitrification Agents (e.g., Liquid Ethane) | Cryogen used for rapid freezing of cryo-EM samples to form a vitreous (non-crystalline) ice layer that preserves the native structure of macromolecules [76]. |
Frequently Asked Questions (FAQs)
FAQ 1: What are the key strengths of AlphaFold2 for membrane protein modeling? AlphaFold2 (AF2) has dramatically expanded the structural coverage of the human transmembrane proteome. It can predict many transmembrane protein structures with high accuracy, particularly when multiple homologous structures exist in the training data. The per-residue confidence metric (pLDDT) is a reliable indicator of local model quality, and for helical transmembrane proteins, AF2 predictions often correctly identify membrane-spanning segments. Furthermore, specialized databases like TmAlphaFold post-process AF2 predictions to embed them within a membrane plane and provide quality assessments based on topological plausibility [82].
FAQ 2: What are the major limitations of AlphaFold2 for predicting membrane protein structures and dynamics? AF2 has several critical limitations for membrane protein applications:
- Static Snapshots: AF2 typically predicts a single, static conformation and struggles to capture multiple biologically relevant states, such as the different conformational states of GPCRs or nuclear receptors [83] [84].
- Fold Switching: It often fails to predict fold-switching proteins, which are sequences that can adopt two distinct stable folds. AF2 typically predicts only one of the conformations, often with high confidence [85].
- Ligand and Environmental Effects: The model is not explicitly aware of the membrane bilayer, ligands, ions, or post-translational modifications. It cannot predict how these factors influence protein structure and stability [86] [84].
- Orphan Proteins: Performance is lower for "orphan" proteins with few sequence homologs, as its predictions rely heavily on evolutionary information from multiple sequence alignments (MSAs) [86].
FAQ 3: How reliable are AlphaFold2's built-in confidence scores (pLDDT and PAE)? The pLDDT score (0-100) is a strong indicator of local backbone reliability. Low pLDDT (< 70) often corresponds to intrinsically disordered regions or areas of high flexibility. The Predicted Aligned Error (PAE) indicates the relative confidence in the spatial relationship between different parts of the structure. Crucially, a high pLDDT does not guarantee the prediction is correct for your biological context, especially for regions known to be involved in conformational changes. The PAE plot is essential for identifying potentially erroneous domain arrangements in multidomain membrane proteins [86] [84].
FAQ 4: My AlphaFold2 model has a low-confidence region in a critical functional domain. What should I do? Low-confidence regions require caution. First, check if the region is a known intrinsically disordered region. If not, this low confidence may signal a limitation in AF2's ability to model this area. You should:
- Seek Experimental Data: Use experimental techniques like cryo-EM, NMR, or HDX-MS to validate or refine the model.
- Investigate Homologs: Run AF2 on close homologs to see if the low confidence is consistent.
- Use Specialized Databases: Consult TmAlphaFold to see if the low-confidence region creates a topological error when the protein is placed in a membrane [82].
FAQ 5: Can I use AlphaFold2 to predict the effect of a point mutation on stability or function? No. The standard AF2 model is not sensitive to point mutations. It works through pattern recognition from evolutionary data, not by simulating physical forces. To assess mutational effects, you must use specialized protein design and stability prediction tools like Rosetta or EvoEF, or methods that use AF2 models as a starting point for molecular dynamics simulations [86].
FAQ 6: What computational tools can I use to design stable membrane protein variants? While de novo design is advanced, a robust approach for stabilizing existing membrane proteins is evolution-guided atomistic design. This method combines:
- Evolutionary Analysis: Filtering design choices to mutations observed in natural homologous sequences to maintain fold stability and avoid aggregation-prone regions.
- Atomistic Calculations: Using physics-based energy functions to stabilize the desired native state. This combined strategy has been successfully used to dramatically improve the thermal stability and heterologous expression yields of challenging membrane proteins, such as the malaria vaccine candidate RH5 [87].
Troubleshooting Guides
Troubleshooting Scenario 1: AlphaFold2 Model Disagrees with Experimental Topology Data
Problem: Your AF2-predicted model for a transmembrane protein shows a different number of transmembrane helices or an incorrect orientation of loops (cytosolic vs. extracellular) compared to experimental topology mapping data.
Diagnosis: This is a known issue where AF2 fails to correctly model the membrane-embedded regions, often due to a lack of evolutionary constraints or specific structural templates in its training set [82].
Solution:
- Consult TmAlphaFold: Input your protein's UniProt ID into the TmAlphaFold database. It provides a quality assessment specifically for membrane proteins, flagging common errors like globular domains mistakenly placed within the membrane [82].
- Validate with pLDDT and PAE: Check the pLDDT scores for the disputed transmembrane regions. Low confidence (pLDDT < 70) supports the likelihood of an error. The PAE plot may show high error between these segments and the rest of the structure [82] [84].
- Integrate Topology Predictors: Use dedicated transmembrane topology prediction tools (e.g., Phobius, TOPCONS, MemBrain) to get a consensus view independent of AF2. Use this consensus to manually evaluate the AF2 model.
- Iterative Modeling with Constraints: If experimental data is available, use it as a spatial constraint in advanced modeling pipelines that integrate AF2 with other tools.
Table: TmAlphaFold Database Quality Filters and Their Meanings [82]
| Filter Code | What It Flags | Potential User Action |
|---|---|---|
| F1 | Conflict with independent topography prediction | Manually verify topology with other tools and literature. |
| F2 | Presence of a signal peptide | Mask the signal peptide region and re-run analysis. |
| F3 | Globular domain is embedded in the membrane | This is a critical error; do not trust the membrane placement. |
| F4 | Disordered region is embedded in the membrane | Likely an artifact; treat the region as flexible. |
| F5 | Low pLDDT region in the membrane | The structure in this region is unreliable. |
Troubleshooting Scenario 2: Predicting Multiple Biologically Relevant Conformations
Problem: Your protein is known to adopt distinct conformational states (e.g., active vs. inactive), but AlphaFold2 predicts only a single, high-confidence structure.
Diagnosis: AF2 is designed to predict the most probable single conformation based on its training data and is inherently limited in capturing conformational heterogeneity, such as fold-switching or ligand-induced changes [85] [83].
Solution:
- Identify Known States: Check the PDB for existing experimental structures of your protein or close homologs in different states (e.g., with bound ligands/G-proteins, or different phosphorylation states).
- Leverage the AF2 Training Set: If a specific conformation is overrepresented in the PDB, AF2 will be biased toward it. Be aware of this bias when interpreting the model for functional studies [85].
- Use Advanced Sampling Techniques: Employ methods that force AF2 to sample alternative conformations. This can involve:
- Input Manipulation: Truncating the protein to isolate flexible domains.
- MSA Manipulation: Using engineered sequence alignments or "shallow" MSAs to reduce evolutionary bias and expose alternative folds [85].
- Supplement with MD Simulations: Use the AF2 model as a starting structure for molecular dynamics (MD) simulations to explore the conformational landscape and energy barriers between states.
Troubleshooting Scenario 3: Designing Soluble and Stable Membrane Protein Analogues
Problem: You need a soluble, stable version of a complex membrane protein fold (e.g., a GPCR) for functional studies or drug screening, but traditional design methods are failing.
Diagnosis: De novo design of complex membrane protein topologies has been a major challenge. However, recent deep learning pipelines have successfully created soluble analogues of membrane protein folds while preserving functional motifs [28].
Solution: Adopt a state-of-the-art deep learning design pipeline that inverts the structure prediction process. The following workflow has been experimentally validated to design soluble analogues of GPCRs, claudins, and rhomboid proteases [28]:
- Backbone Generation: Use an inverted AF2 network (AF2seq) to generate amino acid sequences that are predicted to fold into a desired target membrane protein topology.
- Sequence Optimization: Refine the generated sequences using a protein language model like ProteinMPNN to enhance solubility and stability while maintaining the target fold.
- In-silico Filtering: Filter the designed sequences based on:
- Structural similarity to the target (TM-score > 0.8).
- High AF2 reprediction confidence (pLDDT > 80).
- Novelty relative to natural sequences.
Table: Key Research Reagents and Computational Tools for Membrane Protein Design & Validation
| Tool / Reagent | Type | Primary Function in Validation |
|---|---|---|
| AlphaFold2 | Software / Database | Provides a high-accuracy initial structural model for a given amino acid sequence. |
| TmAlphaFold | Database | Evaluates the plausibility of AF2 models for transmembrane proteins within a lipid bilayer. |
| ProteinMPNN | Software | A deep learning-based protein sequence designer used to optimize sequences for stability and solubility. |
| Rosetta | Software Suite | A versatile tool for protein structure prediction, design, and refinement; useful for stability calculations. |
| Nanodiscs / Liposomes | Experimental Reagent | Membrane mimetics used in cryo-EM and biophysical studies to analyze proteins in a near-native lipid environment [20]. |
| PDBTM / OPM | Database | Curated databases of experimental transmembrane protein structures with defined membrane plane orientations. |
Membrane proteins are critical drug targets, but their inherent hydrophobicity and low abundance make them challenging subjects for proteomic analysis [88] [26]. Successful mass spectrometry (MS) analysis depends heavily on the initial quality and purity of the membrane protein preparation. Contaminants can mask the signals of low-abundance proteins, interfere with peptide identification, and ultimately compromise the biological conclusions of a study [89]. This guide provides troubleshooting resources and validated protocols to help researchers accurately assess purity and identify contaminants in their membrane protein samples, ensuring reliable and reproducible results for drug discovery and basic research.
Troubleshooting Guides and FAQs
Frequently Asked Questions
Q1: What are the most common contaminants in membrane proteomics, and how can I avoid them?
- Keratin: Ubiquitous from skin, hair, and dust. To prevent contamination, always wear a lab coat and clean nitrile gloves, use a hairnet, and thoroughly wipe down workspaces with 70% ethanol before starting work [89].
- Polymer Leachates: From plasticware like tubes and tips. Use mass spectrometry-grade plastics (e.g., Eppendorf) and avoid storing solvents or acids in plastic containers. Use glass scintillation vials and pipettes when working with high acid or solvent concentrations [89].
- Detergents: Common lab detergents like NP-40, Tween, and Triton X-100 are incompatible with MS and can severely interfere with results. While some can be removed with extra steps, Triton and Tween should be avoided entirely. Compatible detergents include SDS, but all buffer components must be disclosed to the MS facility for proper cleanup [89] [90].
- Serum Proteins: Abundant proteins like albumin from fetal calf/bovine serum (FCS/BSA) can dominate the signal. For secretome analysis or when working with cultured cells, wash cells three times with PBS prior to lysis and culture in serum-free medium [91].
Q2: My membrane protein yields are low after enrichment. What methods can improve recovery?
Membrane protein yields are often low due to their hydrophobic nature and complex interactions with lipid bilayers [26]. The table below compares common enrichment techniques, highlighting their effectiveness in improving purity and yield for MS analysis.
Table 1: Comparison of Plasma Membrane Protein Enrichment Techniques
| Enrichment Technique | Total Proteins Identified (Range) | Plasma Membrane Purity (Range) | Key Advantages | Key Limitations |
|---|---|---|---|---|
| Whole Cell Lysate [92] | 84 - 112 | 9% - 13% | Most unbiased; minimal sample manipulation. | Low purity; high background of soluble proteins. |
| Crude Membrane Preparation [92] | 104 - 111 | 17% - 20% | Simple centrifugation; reduces soluble protein complexity. | Contains biological contaminants from other organelles. |
| NHS-SS-Biotinylation & Pulldown [92] | 78 - 115 | 27% - 31% | Targets surface-exposed proteins. | May label proteins nonspecifically; efficiency depends on biotinylation. |
| Amino-oxy-biotin Glycoprotein Pulldown [92] | 120 | 65% | Highly selective for sialylated plasma membrane proteins; high purity. | Targets only a specific subpopulation of membrane proteins. |
Q3: Which detergents are mass spectrometry-compatible for membrane protein solubilization?
The choice of surfactant is critical for both extracting membrane proteins from the lipid bilayer and maintaining their stability for analysis [93].
- For MS Sample Preparation: SDS is a commonly used and MS-compatible detergent for cell lysis and initial solubilization [91] [89]. Acid-labile surfactants are also excellent alternatives as they can be easily cleaved before MS analysis [89].
- For Structural Studies (e.g., Cryo-EM): While SDS is used for extraction, it is often replaced with more mild detergents (e.g., DDM, GDN) or detergent-free systems like styrene-maleic acid (SMA) or diisobutylene-maleic acid (DIBMA) copolymers for the final vitrification step. These polymers directly solubilize membrane proteins within their native lipid environment, forming SMA lipid particles (SMALPs), which preserve stability and function [26] [93].
Q4: How much sample input is required for a full membrane proteome analysis?
For a standard full proteome analysis via LC-MS/MS, core facilities typically request 20 µg of protein from cell lysates based on a BSA-calibrated Bradford or BCA assay [91]. For more specialized analyses, requirements vary:
- Immunoprecipitation/Pull-downs: Submit 60 µL of eluate, with equal protein amounts or cell numbers as starting material [91].
- Phosphoproteomics: Requires significantly more input, typically 500-1000 µg of total protein, due to the low stoichiometry of phosphorylation [91].
- Gel Bands: Visible bands on a Coomassie-stained gel generally have a >95% success rate for identification [90].
Troubleshooting Common Problems
Table 2: Troubleshooting Guide for Membrane Protein Proteomics
| Problem | Potential Causes | Solutions |
|---|---|---|
| Low coverage of transmembrane domains | High hydrophobicity of peptides; inefficient digestion and extraction [88]. | Use MS-compatible detergents (e.g., SDS) [89]; incorporate urea washes to enhance identification of multi-spanning proteins [94]; raise LC temperature (e.g., to 60°C) to improve chromatography [88]. |
| High background of non-membrane proteins | Incomplete enrichment; co-isolation of soluble proteins [92]. | Implement a rigorous membrane enrichment protocol (e.g., ultracentrifugation followed by urea or alkaline wash) [94]; use quantitative methods like isotopic labeling to distinguish true membrane proteins from contaminants [88]. |
| Keratin contamination overwhelming signal | Contamination from user, dust, or lab surfaces [89]. | Wear a lab coat, gloves, and hairnet; clean benches and tools with 70% ethanol; maintain separate, clean reagent stocks for MS work. |
| Poor peptide signal or failed run | MS-incompatible detergents (Triton, Tween) in final sample; plasticizer leaching [89]. | Avoid incompatible detergents in lysis buffers; use MS-grade plasticware; store solvents and acids in glass, not plastic. |
Experimental Protocols for Purity Assessment
Protocol 1: Urea Wash for In-Depth Membrane Proteome Analysis
This protocol significantly enhances the identification of integral membrane proteins, particularly multi-spanning transporters and receptors [94].
- Membrane Isolation: Homogenize cells or tissue in an appropriate hypotonic buffer. Isolate the crude membrane fraction via ultracentrifugation (e.g., 200,000 × g for 1.5 hours) [92].
- Urea Wash: Resuspend the membrane pellet in a buffer containing 6 M Urea. Incubate for a set time, then recover the washed membranes by another round of ultracentrifugation.
- Solubilization and Digestion: Solubilize the final membrane pellet in an MS-compatible lysis buffer containing SDS. Proceed with standard protein quantification and digestion protocols, such as Filter-Aided Sample Preparation (FASP) [92] or the SP3 protocol [91].
- MS Analysis: Analyze the resulting peptides by LC-MS/MS.
Outcome: This method has been shown to identify almost twice as many membrane proteins compared to non-enriched samples and can enhance the identification of multi-spanning transmembrane proteins by almost sixfold [94].
Protocol 2: Selective Biotinylation for High-Purity Plasma Membrane Isolation
This method uses amino-oxy-biotin to selectively label and isolate sialylated cell surface glycoproteins, resulting in a highly pure plasma membrane fraction [92].
- Oxidation: Wash cells in ice-cold PBS. Incubate cells with 1 mM sodium meta-periodate to oxidize surface sialic acid residues. Quench the reaction with glycerol.
- Biotinylation: Label the oxidized residues with 100 mM amino-oxy-biotin and 10 mM aniline in PBS, pH 6.7, for 1 hour at 4°C.
- Lysis and Pull-down: Lyse biotinylated cells in a Triton X-100-based lysis buffer. Remove nuclei by centrifugation. Incubate the supernatant with high-affinity streptavidin agarose beads to capture biotinylated proteins.
- Stringent Washing: Wash the beads extensively with lysis buffer, followed by PBS with 0.5% SDS, and then urea-based buffers to remove non-specifically bound contaminants.
- On-Bead Digestion: Digest the captured glycoproteins directly on the beads with trypsin for MS analysis.
Outcome: This technique can achieve a plasma membrane purity of 65%, identifying 120 proteins with high confidence. When combined with peptide fractionation techniques like Strong Anion Exchange (SAX), identifications can increase to 281 proteins (54% purity) [92].
Workflow Visualization
The following diagram illustrates the logical workflow for assessing the purity of a membrane protein preparation, from initial isolation to final MS analysis and data interpretation.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents for Membrane Protein Proteomics
| Reagent / Material | Function / Application | Key Considerations |
|---|---|---|
| SDS (Sodium Dodecyl Sulfate) [91] [89] | Harsh ionic detergent for complete cell lysis and membrane protein solubilization. | MS-compatible; can be used in initial steps but may need removal or replacement for downstream steps. |
| Urea [94] | Chaotrope used to wash isolated membrane fractions, removing peripheral proteins. | Significantly increases identification of multi-spanning transmembrane domains. |
| Amino-oxy-biotin [92] | Selective biotinylation reagent for sialylated glycoproteins on the cell surface. | Enables highly specific enrichment of plasma membrane proteins; achieves high purity. |
| Styrene-Maleic Acid (SMA) [26] | Amphiphilic copolymer for detergent-free extraction of membrane proteins. | Forms SMALPs, preserving the native lipid bilayer around the protein for enhanced stability. |
| Mass Spectrometry-Grade Plastics [89] | Tubes and tips tested to prevent polymer leaching. | Critical for avoiding chemical contaminants that can dominate the MS signal. |
| Protease/Phosphatase Inhibitors [91] | Prevent protein degradation and preserve post-translational modifications during lysis. | EDTA-free cocktails are typically recommended for compatibility with MS workflows. |
Frequently Asked Questions (FAQs)
FAQ 1: What is the difference between specific lipid binding and preferential lipid solvation, and why does it matter for my functional assays?
Specific lipid binding involves long-lived interactions where a lipid acts as a classical ligand at a defined protein site, often showing saturation at high concentrations. In contrast, preferential lipid solvation is a dynamic effect where certain lipid types become enriched around a protein without forming long-lived complexes, instead influencing protein conformational equilibria through the thermodynamics of the solvation shell. This distinction is critical because it determines your validation strategy; solvation effects do not saturate and require techniques that measure changes in conformational stability within a bilayer rather than just identifying co-purifying lipids [11] [95].
FAQ 2: My membrane protein preparation co-purifies with lipids. How can I determine if these lipids are specific binders or just the most abundant in the membrane?
Simply identifying co-purifying lipids can be misleading, as this can reflect the extraction process rather than functional specificity [11]. To determine true specificity:
- Perform lipid exchange experiments using methods like Lipid Exchange-Mass Spectrometry (LX-MS), which measures a protein's affinity for individual lipids from a complex mixture against a reference nanodisc [96].
- Conduct equilibrium titration studies within a defined lipid bilayer. If the lipid effect on your protein's activity or oligomerization state does not saturate with increasing lipid concentration, it strongly indicates a preferential solvation mechanism rather than specific binding [11].
FAQ 3: I have observed a lipid-dependent functional effect in my reconstituted system. What is the first step to validate that this regulation is native-like?
The first step is to replicate the functional assay in a system that more closely mimics the native membrane's chemical complexity. Instead of using a single synthetic lipid, reconstitute your protein into native lipid extracts or defined mixtures that contain the suspected regulatory lipid alongside other lipid types known to be present in vivo. This controls for the phenomenon of coincidence detection, where robust membrane localization and function often require two distinct lipid species [97]. Observing the same functional effect in a complex environment strengthens the case for its biological relevance.
Troubleshooting Guide
Problem 1: Inconsistent Lipid Regulation Observed Between Native Membranes and Synthetic Liposomes
| Symptoms | Possible Causes | Recommended Solutions |
|---|---|---|
| Protein shows functional regulation in cell-derived membranes but not in synthetic bilayers of defined composition [97]. | The synthetic system lacks essential lipid co-factors for coincidence detection. | - Screen for functional recovery by systematically adding minor lipid fractions (e.g., PS, PA, PI) to your base PC lipid [97].- Use native mass spectrometry with natural lipid extracts to identify a profile of lipids that co-purify with the protein, then reconstitute using a synthetic blend based on this profile [98]. |
| The physical-chemical properties (e.g., membrane packing, curvature) of the synthetic bilayer do not match the native environment. | - Incorporate lipids with similar intrinsic curvature and adjust the ratio of saturated/unsaturated acyl chains to better match the packing density of native membranes [99].- Use shotgun lipidomics on native membranes to determine the relevant acyl chain profile and mimic it synthetically [99]. |
Problem 2: Difficulty in Distinguishing Specific Lipid Binders from Non-Specific Solvation Effects
| Symptoms | Possible Causes | Recommended Solutions |
|---|---|---|
| Structures or mass spectrometry show associated lipids, but functional assays show no dependence on these lipids [11]. | The observed lipids may be non-specific, trapped during purification, or represent preferential solvation rather than specific binding. | - Use lipidomic LX-MS: incubate protein in nanodiscs with a complex lipid extract alongside empty nanodiscs. After exchange, identify lipids specifically enriched in protein-containing nanodiscs via mass spectrometry [96].- Perform a titration experiment: if the functional effect (e.g., on dimerization) shows no saturation and is detectable even at very low mole fractions (<1%) of the regulatory lipid, it indicates preferential solvation [11] [95]. |
Problem 3: Poor Reproducibility of Lipid-Dependent Oligomerization or Conformational Shifts
| Symptoms | Possible Causes | Recommended Solutions |
|---|---|---|
| The observed population of protein dimers or a specific conformational state varies significantly between preparations. | Inconsistent lipid composition between reconstitutions leads to shifts in the protein's conformational equilibrium. | - Strictly control the lipid-to-protein ratio and lipid composition during reconstitution.- Use a single-molecule assay (e.g., single-molecule FRET in liposomes) to directly quantify the populations of different conformational states within a heterogeneous sample [11].- Employ coarse-grained molecular dynamics (CGMD) simulations to model how different lipid compositions affect the solvation free energy of the monomeric vs. oligomeric state, providing a theoretical basis for your experimental observations [11]. |
Essential Experimental Protocols
Protocol 1: Lipidomic Lipid Exchange-Mass Spectrometry (LX-MS)
Purpose: To quantitatively identify lipids with genuine affinity for a membrane protein from a complex, native-like lipid mixture [96].
Workflow:
- Prepare Membrane Protein Nanodiscs: Incorporate the target membrane protein into nanodiscs using a native lipid extract (e.g., E. coli polar lipid extract or brain polar lipid extract).
- Prepare Empty Nanodiscs: Prepare identical nanodiscs but without the embedded membrane protein.
- Incubate for Passive Exchange: Mix the two populations of nanodiscs and incubate to allow lipids to passively exchange between them.
- Separate Nanodisc Populations: Use a separation technique (e.g., affinity chromatography) to isolate the membrane protein nanodiscs from the empty nanodiscs after exchange.
- Lipidomic Analysis: Extract lipids from both populations and analyze them using liquid chromatography-mass spectrometry (LC-MS).
- Data Analysis: Calculate the enrichment of each lipid species in the protein-containing nanodiscs compared to the empty nanodiscs. Significant enrichment indicates specific binding affinity [96].
The following diagram illustrates this multi-step workflow.
Protocol 2: Validating Preferential Solvation via Dimerization Equilibrium Titration
Purpose: To determine if a lipid's influence on a membrane protein's oligomerization state occurs via specific binding or dynamic preferential solvation [11] [95].
Workflow:
- Reconstitute Protein in Reference Bilayer: Incorporate the protein (e.g., CLC-ec1) into a well-defined lipid bilayer (e.g., POPC) and establish a baseline dimerization constant.
- Titrate in Candidate Lipid: Titrate in a second, chemically distinct lipid (e.g., short-chain DLPC) across a broad concentration range, from very low (e.g., <1 mol%) to high mole fractions.
- Measure Dimerization Constant: At each titration point, use a quantitative technique (e.g., single-molecule fluorescence, analytical ultracentrifugation) to measure the resulting dimerization constant.
- Analyze Binding Isotherm:
- Saturation Profile: If the lipid acts as a classic ligand, the change in dimerization free energy will plateau (saturate) as specific binding sites are filled.
- Linear/Non-Saturating Profile: If the effect is linear and shows no saturation, even at low concentrations, it is indicative of a non-specific, thermodynamic solvation effect [11].
This mechanistic distinction is crucial for data interpretation.
Research Reagent Solutions Toolkit
The following table details key reagents and their applications in studying protein-lipid interactions.
| Reagent / Material | Function in Lipid Interaction Studies | Key Application Notes |
|---|---|---|
| Native Lipid Extracts (e.g., E. coli polar, brain polar) | Provides a natural, complex lipid milieu for identifying physiologically relevant protein-lipid interactions [96] [98]. | Essential for discovery-based approaches like LX-MS and native MS to avoid bias from synthetic lipid systems [96]. |
| Nanodiscs (MSP / SMA) | Creates a controlled, native-like membrane environment for solubilizing membrane proteins for various biophysical analyses [96]. | Allows for precise control of the lipid-to-protein ratio and is compatible with LX-MS, SPR, and other solution-based techniques [97] [96]. |
| Short-Chain Lipids (e.g., DLPC, DLPG) | Acts as a molecular probe for preferential solvation effects due to their ability to efficiently solvate regions of hydrophobic mismatch [11]. | A non-saturating inhibitory effect on oligomerization at low concentrations is a hallmark of preferential solvation [11] [95]. |
| Surface Plasmon Resonance (SPR) Sensor Chips (e.g., L1 chip) | Used to measure kinetic parameters (Ka, Kd) of protein binding to liposomes in real-time [97]. | Considered the "gold standard" for quantitative affinity measurements but requires careful optimization to minimize nonspecific binding [97]. |
| C-Laurdan Dye | A solvatochromic dye that reports on membrane packing and order by measuring general polarization (GP) [99]. | Used to validate whether lipidomic remodeling in response to a perturbation (e.g., PUFA supplementation) successfully restores membrane biophysical properties [99]. |
The table below summarizes key quantitative methods for validating lipid interactions, helping you choose the right tool for your research question.
| Technique | Measured Parameter | Information Gained | Throughput |
|---|---|---|---|
| Lipidomic LX-MS [96] | Lipid Enrichment Factor | Binding affinity and specificity for hundreds of lipids from a natural extract simultaneously. | Medium |
| Surface Plasmon Resonance (SPR) [97] | Association/Dissociation Rate Constants (ka, kd), Equilibrium Binding Constant (Kd) | Quantitative kinetics and affinity of protein binding to lipid surfaces or specific lipids. | Low |
| Native MS with Lipidomics [98] | Mass of intact protein-lipid complexes; identity of released lipids | Identifies specific lipid species that remain bound to the protein through purification. | Low |
| Single-Molecule Fluorescence [11] | Stoichiometry, Conformational/Dimerization Population Distributions | Quantifies the equilibrium populations of protein states within a lipid bilayer. | Low |
| Isothermal Titration Calorimetry (ITC) [97] | Enthalpy (ΔH), Entropy (ΔS), Binding Stoichiometry (N), Kd | Full thermodynamic profile of the binding interaction. | Low |
Conclusion
The quality assessment of membrane protein preparations is a multi-faceted process that integrates foundational knowledge, rigorous methodological application, systematic troubleshooting, and advanced validation. As the field advances, the convergence of biophysical techniques, computational predictions, and a deeper understanding of lipid-protein interactions will set new standards for preparation quality. These advancements are pivotal for accelerating the structure-function elucidation of membrane proteins, ultimately driving innovation in drug discovery and the development of therapeutics for a wide range of diseases. Future directions will likely focus on high-throughput screening methods, the design of more effective membrane mimetics, and the integration of artificial intelligence to predict optimal purification and stabilization conditions.
References
- 1. Developing a High-Quality Scoring Function for Membrane ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3322274/]
- 2. Evaluation of Transmembrane Protein Structural Models ... [https://www.mdpi.com/2673-7426/3/2/21]
- 3. Troubleshooting: Protein Expression [https://www.goldbio.com/blogs/articles/troubleshooting-protein-expression?srsltid=AfmBOoqO5ykH9z2mFfLZUfo-lQcTN40LyUxF8vUQVhAsyjzTsPKRBy3_]
- 4. Avoid Common Obstacles in Protein Expression [https://www.neb.com/en-us/tools-and-resources/feature-articles/bypassing-common-obstacles-in-protein-expression?srsltid=AfmBOorwqXlDLLLLEw2-GR0TRVjO3kMgs6BoE04ibfkbQG6F2B9gynmc]
- 5. Working with Membrane Proteins: 12 Simple Tips for Success [https://bitesizebio.com/52681/working-with-membrane-proteins/]
- 6. How to Make Membrane Protein Research Less Frustrating [https://www.fidabio.com/blogs/how-to-make-membrane-protein-research-less-frustrating]
- 7. Four Reasons Why Your Membrane Protein Expression ... [https://www.leniobio.com/rescue-failing-membrane-protein-expression/]
- 8. Membrane Protein Stabilization Strategies for Structural ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7926488/]
- 9. Stabilizing membrane proteins [https://www.sciencedirect.com/science/article/abs/pii/S0959440X00002232]
- 10. The Peptidisc, a simple method for stabilizing membrane ... [https://elifesciences.org/articles/34085]
- 11. Molecular basis for the regulation of membrane proteins ... [https://www.nature.com/articles/s41589-025-02032-w]
- 12. Protein stability governed by its structural plasticity is ... [https://www.nature.com/articles/s41598-020-58825-7]
- 13. Avoid Common Obstacles in Protein Expression [https://www.neb.com/en-us/tools-and-resources/feature-articles/bypassing-common-obstacles-in-protein-expression?srsltid=AfmBOopyI61Rd3Jc9ohqpoy75QycZ45dgH9uAbHGKYXJXsJj0YWrCVbW]
- 14. Non-invasive tools for analysis of plasma membrane ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12103270/]
- 15. Why Membrane Proteins Matter - And Why They're So Hard ... [https://peakproteins.com/why-membrane-proteins-matter-and-why-theyre-so-hard-to-study/]
- 16. Helical Membrane Protein Conformations and their ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3818118/]
- 17. Advances and challenges in preparing membrane proteins ... [https://www.sciencedirect.com/science/article/abs/pii/S0734975024001770]
- 18. Protein Purificaiton Troubleshooting & Best Practices [https://www.bostonind.com/blog/protein-purificaiton-troubleshooting-and-best-practices?srsltid=AfmBOoqajuuRAGRhDEjcNopmA688M5f4gZCIfbzwBNtSZc2Ozi1Qiiw2]
- 19. The lipid bilayer strengthens the cooperative network of ... [https://pubmed.ncbi.nlm.nih.gov/40601723/]
- 20. Interactions between membrane proteins and lipid ... [https://www.biophysics-reports.org/article/doi/10.52601/bpr.2025.240073]
- 21. Global and local effects in lipid-mediated interactions ... [https://www.frontiersin.org/journals/molecular-biosciences/articles/10.3389/fmolb.2025.1605772/full]
- 22. Simulations reveal a balance between protein–protein and ... [https://pubs.rsc.org/en/content/articlehtml/2025/sc/d5sc04862a]
- 23. Quantitative imaging of lipid transport in mammalian cells [https://www.nature.com/articles/s41586-025-09432-x]
- 24. Coming Clean and Avoiding Bubble Trouble–Using ... [https://www.mdpi.com/2218-273X/15/9/1315]
- 25. DeFrND: detergent-free reconstitution into native ... [https://www.nature.com/articles/s41467-025-63275-8]
- 26. Advances in solubilization and stabilization techniques for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11974516/]
- 27. Membrane-mimetic thermal proteome profiling (MM-TPP) ... [https://elifesciences.org/articles/104549]
- 28. Computational design of soluble and functional membrane ... [https://www.nature.com/articles/s41586-024-07601-y]
- 29. Rapid Assessment Of Membrane Protein Quality [https://peakproteins.com/rapid-assessment-of-membrane-protein-quality-by-fluorescent-size-exclusion-chromatography/]
- 30. Best Practices for Aggregate Quantitation of Antibody ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9232890/]
- 31. Analytical Ultracentrifugation (AUC) - PubMed Central - NIH [https://pmc.ncbi.nlm.nih.gov/articles/PMC7781197/]
- 32. Analytical Ultracentrifugation: A Comprehensive Overview [https://www.bostonind.com/blog/analytical-ultracentrifugation-a-comprehensive-overview?srsltid=AfmBOorPl-yd6CKeu4BHZoOCytfdAzyApmD9d4cvrRAd8FFN0iAQXf1A]
- 33. Analytical ultracentrifugation (AUC) [https://www.physiolchemie.abi.med.uni-muenchen.de/biophysics/equipment/auc/index.html]
- 34. The rapid “teabag” method for high-end purification of ... [https://www.nature.com/articles/s41598-020-73285-9]
- 35. Rapid Assessment of Membrane Protein Quality by ... [https://pubmed.ncbi.nlm.nih.gov/36688542/]
- 36. Moving analytical ultracentrifugation software to a good ... [https://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1007942]
- 37. Tips & Tricks GPC/SEC: Column Issues with Light ... [https://www.chromatographyonline.com/view/tips-tricks-gpcsec-column-issues-light-scattering-detectors]
- 38. Quality assessment and optimization of purified protein samples [https://pmc.ncbi.nlm.nih.gov/articles/PMC4299812/]
- 39. Protein Homogeneity [https://www.creative-biostructure.com/protein-homogeneity.htm?srsltid=AfmBOorhJpAvCV4iN6vHLUkuNekUGmPoulV_quRo9XL09IgiZKXPhY25]
- 40. An efficient method for detecting membrane protein ... [https://pubmed.ncbi.nlm.nih.gov/38332570]
- 41. HPLC Troubleshooting Guide [https://scioninstruments.com/us/blog/hplc-troubleshooting-guide-2/]
- 42. HPLC Troubleshooting Guide [https://www.thermofisher.com/us/en/home/industrial/chromatography/chromatography-learning-center/high-performance-liquid-chromatography-hplc-support/hplc-troubleshooting.html]
- 43. Troubleshooting - Immunofluorescence Assays [https://ibidi.com/content/366--troubleshooting]
- 44. Immunofluorescence Troubleshooting | Tips & Tricks [https://www.stressmarq.com/support/technical-support/troubleshooting/immunofluorescence-troubleshooting/?srsltid=AfmBOoplOVA0SKJAE4RUg5ZsiLU_pacYHBI-35YZKiD3OWtVzooanpbH]
- 45. Immunofluorescence (IF) Troubleshooting Guide [https://www.cellsignal.com/learn-and-support/troubleshooting/if-troubleshooting-guide?srsltid=AfmBOoq9Y4PdPnh-zRBFvEfo4oIJljoOI5os8aXJZucXLhndQoMctFxZ]
- 46. ELISA Troubleshooting Guide [https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overview-elisa/elisa-troubleshooting-guide.html]
- 47. Flow Cytometry Protocol | Abcam [https://www.abcam.com/en-us/technical-resources/protocols/flow-cytometry-for-intracellular-and-extracellular-targets]
- 48. Testing and characterizing enzymes and membrane-bound ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC1132645/]
- 49. Functional Assay Protocol & Troubleshooting - Creative Biolabs [https://www.creativebiolabs.net/functional-assay.htm]
- 50. Biophysical methods in early drug discovery - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC8963580/]
- 51. Standard operating procedure combined with ... [https://www.nature.com/articles/s41467-025-56337-4]
- 52. Membrane Filtration-Assisted Enzymatic Hydrolysis Affects ... [https://www.mdpi.com/1420-3049/26/4/852]
- 53. Transport Assay - an overview [https://www.sciencedirect.com/topics/nursing-and-health-professions/transport-assay]
- 54. Membrane Protein Solubilization [https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/protein-biology/protein-purification/detergent-solubilization-of-membrane-proteins?srsltid=AfmBOoonIhDyjzysfA4T0EkzLT57q1bFPTr5II0mC-Coo2sDQ4uwfKhC]
- 55. Exploring the World of Membrane Proteins: Techniques ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10608922/]
- 56. High-throughput stability screening for detergent ... [https://www.nature.com/articles/s41598-019-46686-8]
- 57. Detergent-mediated protein aggregation - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC5007131/]
- 58. Video: Rapid Assessment of Membrane Protein Quality by ... [https://www.jove.com/v/64322/rapid-assessment-membrane-protein-quality-fluorescent-size-exclusion]
- 59. PMC - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC12466997/]
- 60. 6 Easy Ways to Remove Excess Detergent from Membrane ... [https://bitesizebio.com/53887/remove-excess-detergent-from-membrane-proteins/]
- 61. Protein Assays and Quantitation Support—Troubleshooting [https://www.thermofisher.com/us/en/home/technical-resources/technical-reference-library/protein-assays-analysis-support-center/protein-assays-quantitation-support/protein-assays-quantitation-support-troubleshooting.html]
- 62. Propidium Iodide Cell Viability Flow Cytometry Protocol [https://www.rndsystems.com/resources/protocols/flow-cytometry-protocol-analysis-cell-viability-using-propidium-iodide]
- 63. Cell cycle analysis with flow cytometry and propidium iodide [https://www.abcam.com/en-us/technical-resources/protocols/flow-cytometry-with-propidium-iodide-staining]
- 64. Propidium Iodide Applications & Common Issues [https://www.aatbio.com/resources/application-notes/propidium-iodide-applications-common-issues]
- 65. Confocal Microscopy Provides Powerful Imaging Insights [https://www.the-scientist.com/confocal-microscopy-provides-powerful-imaging-insights-73485]
- 66. Flow Cytometry Troubleshooting Guide [https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/protein-biology/flow-cytometry/flow-cytometry-troubleshooting-guide?srsltid=AfmBOopvuY1vnDFk8NExLdyc0nHLXXiq-JVPHHIYDg_L0cQEiyxAk_3Z]
- 67. Troubleshooting | Cytometry [https://cytometry.mlsascp.com/troubleshooting.html]
- 68. Mammalian Cell Culture Basics Support—Troubleshooting [https://www.thermofisher.com/us/en/home/technical-resources/technical-reference-library/cell-culture-support-center/mammalian-cell-culture-basics-support/mammalian-cell-culture-basics-support-troubleshooting.html]
- 69. Evaluation of protein extraction methodologies on bacterial ... [https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2025.1586662/full]
- 70. Mastering Mammalian Cell Culture Maintenance: A Guide ... [https://cellculturecompany.com/mastering-mammalian-cell-culture-maintenance-a-guide-for-biomedical-researchers/]
- 71. Advances in Recovery and Purification - Part 2 - 2025 [https://www.bioprocessingeurope.com/25/purification-part-2]
- 72. Smart culture medium optimization for recombinant protein ... [https://www.sciencedirect.com/science/article/abs/pii/S0734975025002241]
- 73. Protocol for plasma membrane enrichment by ... [https://www.sciencedirect.com/science/article/pii/S2666166725004551]
- 74. Optimization of a novel expression system for recombinant ... [https://www.nature.com/articles/s41598-024-76995-6]
- 75. Sustainable Protein Recovery and Wastewater Valorization ... [https://www.mdpi.com/2304-8158/14/12/2044]
- 76. X-rays in the Cryo-EM Era: Structural Biology's Dynamic Future [https://pmc.ncbi.nlm.nih.gov/articles/PMC5999524/]
- 77. Membrane Protein Structural Validation by Oriented ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4008839/]
- 78. Validation, analysis and annotation of cryo-EM structures [https://pmc.ncbi.nlm.nih.gov/articles/PMC8411978/]
- 79. Validation and Quality Assessment of X-ray Protein Structures [https://www.proteinstructures.com/protein-structure-validation/]
- 80. Validation and quality assessment of macromolecular ... [https://www.nature.com/articles/s41598-019-38658-9]
- 81. Protein Structure Analysis and Validation with X-Ray ... [https://pubmed.ncbi.nlm.nih.gov/33128762/]
- 82. How AlphaFold2 shaped the structural coverage of ... [https://www.nature.com/articles/s41598-023-47204-7]
- 83. Bridging prediction and reality: Comprehensive analysis of ... [https://www.sciencedirect.com/science/article/pii/S2001037025001734]
- 84. The power and pitfalls of AlphaFold2 for structure prediction ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11956457/]
- 85. AlphaFold2 fails to predict protein fold switching - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9134877/]
- 86. Strengths and limitations of AlphaFold 2 [https://www.ebi.ac.uk/training/online/courses/alphafold/an-introductory-guide-to-its-strengths-and-limitations/strengths-and-limitations-of-alphafold/]
- 87. Opportunities and challenges in design and optimization of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7616297/]
- 88. Mass Spectrometry Accelerates Membrane Protein Analysis [https://pmc.ncbi.nlm.nih.gov/articles/PMC3222592/]
- 89. Common Mass Spectrometry Contaminants: I Messed It Up ... [https://bitesizebio.com/26879/common-mass-spectrometry-contaminants-messed-dont/]
- 90. Proteomics and Metabolomics FAQs [https://medschool.duke.edu/research/research-support/core-facilities-service-centers/find-service-center/proteomics-and-5]
- 91. FAQs – Proteomics Core Facility [https://www.embl.org/groups/proteomics/faqs/]
- 92. Comparative Analysis of Techniques to Purify Plasma ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2922835/]
- 93. Overview of Membrane Protein Sample Preparation for ... [https://www.mdpi.com/1422-0067/24/19/14785]
- 94. Simple But Efficacious Enrichment of Integral Membrane ... [https://www.sciencedirect.com/science/article/pii/S1535947622000147]
- 95. Molecular basis for the regulation of membrane proteins ... [https://pubmed.ncbi.nlm.nih.gov/41006774/]
- 96. Identifying Membrane Protein-Lipid Interactions with Lipidomic ... [https://pubmed.ncbi.nlm.nih.gov/37700579/]
- 97. Emerging methodologies to investigate lipid–protein ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3722868/]
- 98. Combining native mass spectrometry and lipidomics to ... - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10430552/]
- 99. Lipidomic and biophysical homeostasis of mammalian ... [https://www.nature.com/articles/s41467-020-15203-1]